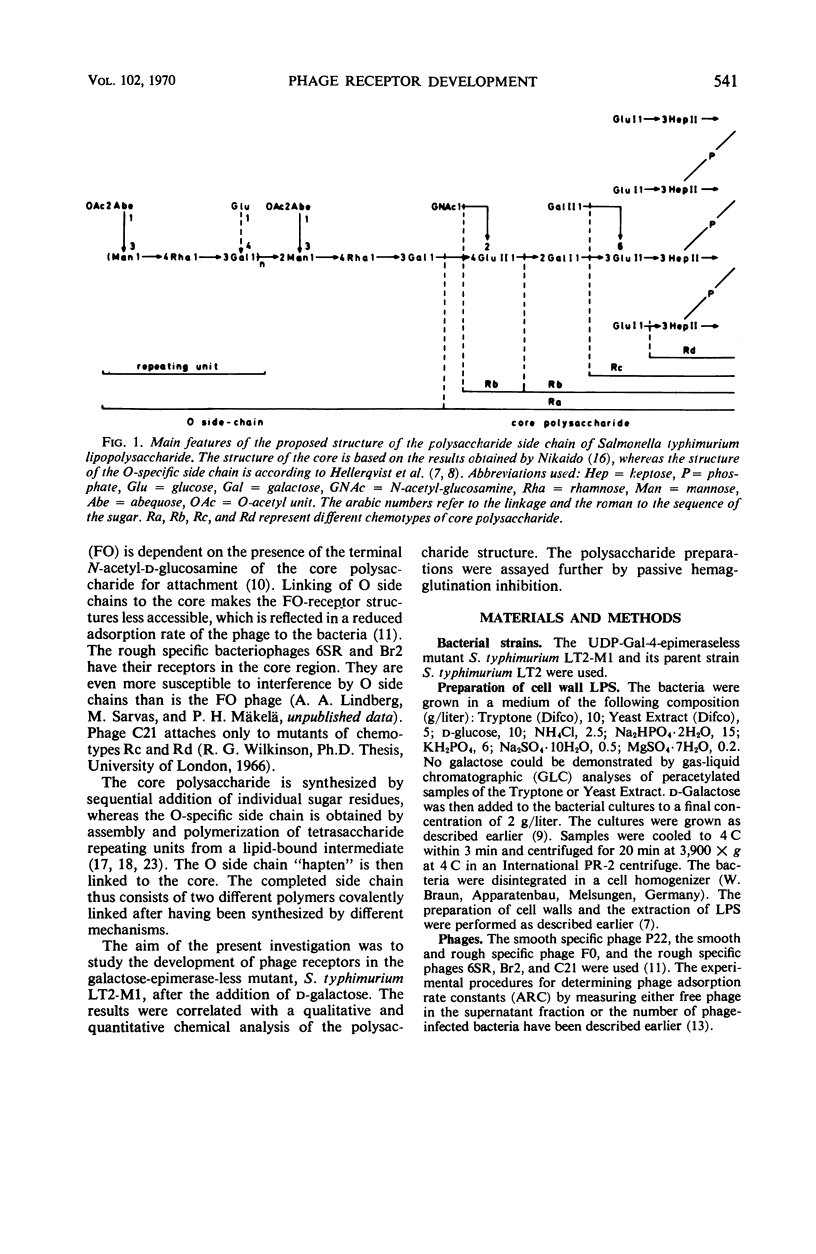
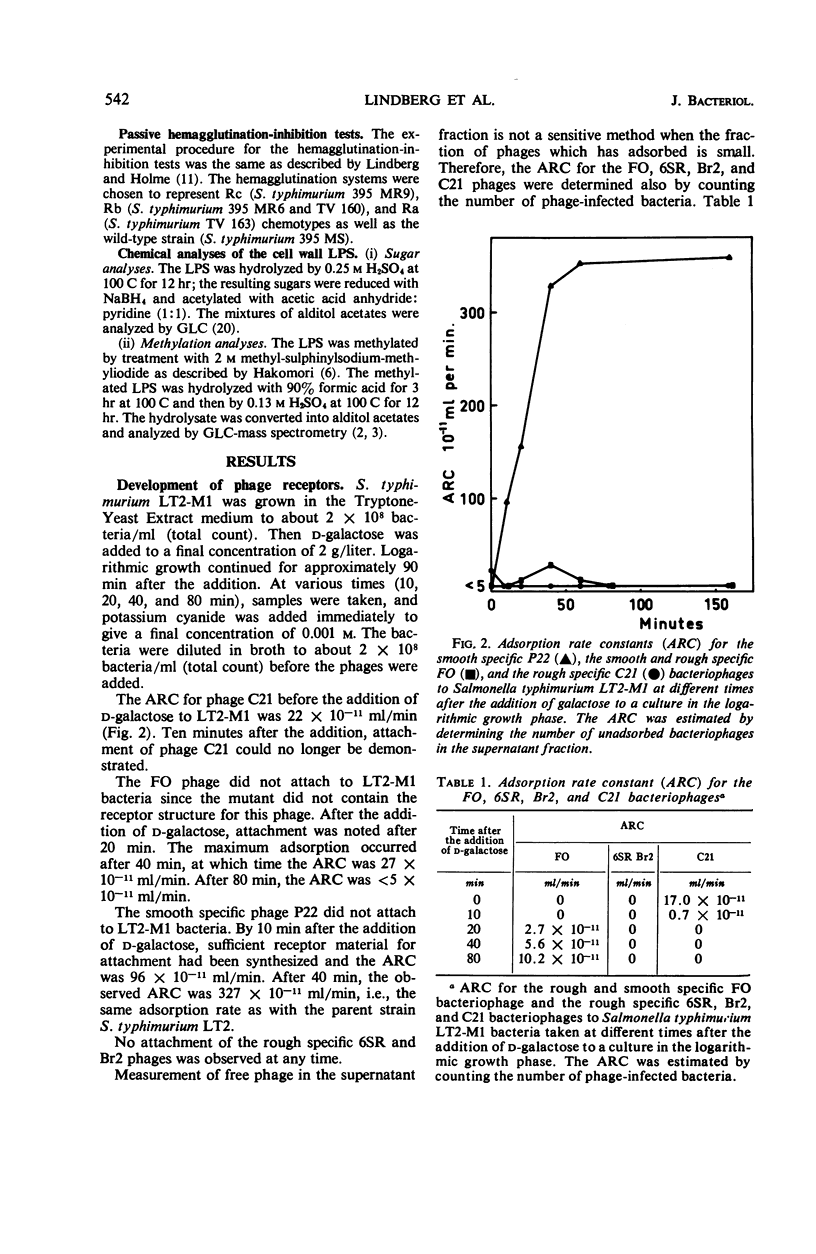
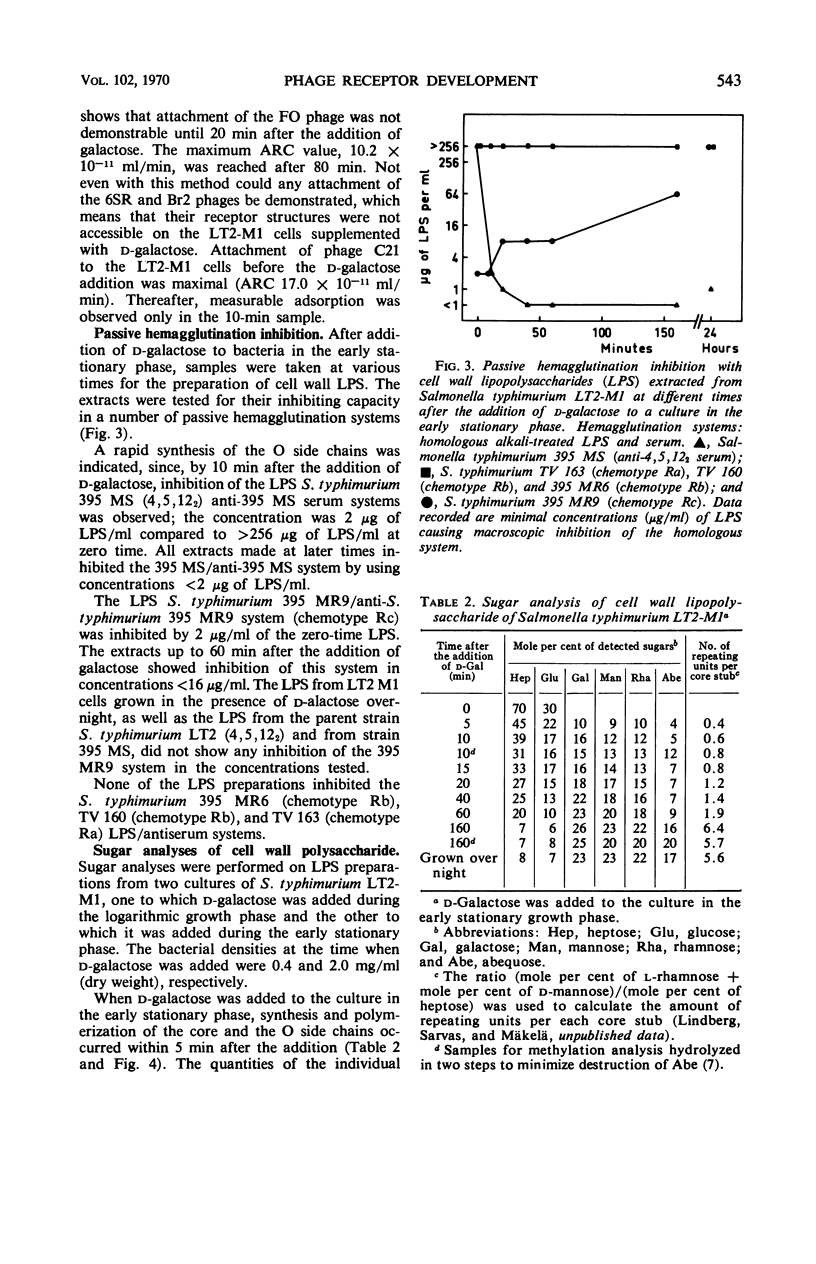
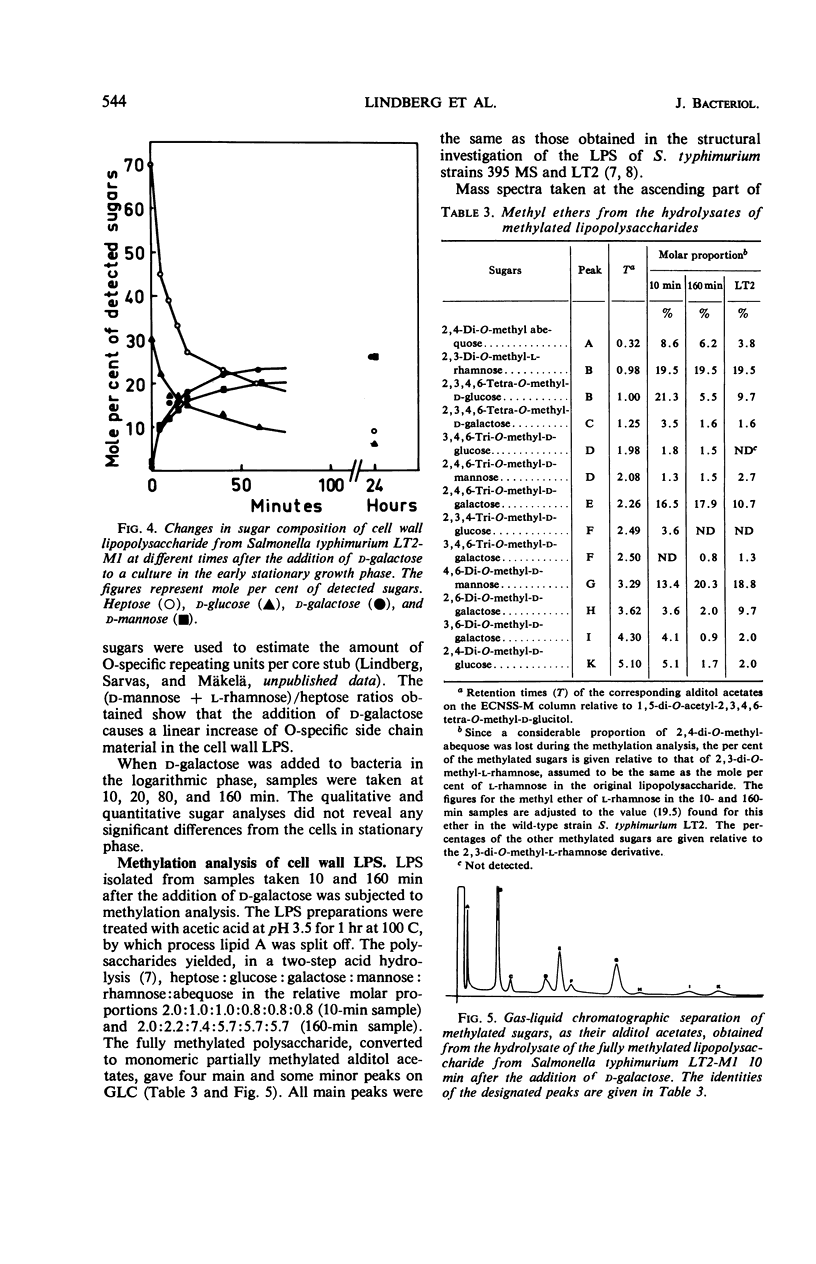
Abstract
The formation of complete cell wall core lipopolysaccharide (LPS) and O-antigenic side chains after addition of d-galactose to the uridine diphosphate-galactose-4-epimeraseless mutant, Salmonella typhimurium LT2-M1, has been studied by (i) determination of adsorption rates of smooth and rough specific bacteriophages, (ii) passive hemagglutination inhibition, and (iii) qualitative and quantitative determination of the polysaccharide composition and structure. A rapid synthesis of the complete core LPS and O side chains occurred in bacteria in the log phase and the early stationary phase. Phage C21, which attaches to unsubstituted Rc structures, was adsorbed by the bacteria for only 10 min after the addition of d-galactose. Unsubstituted Rc structures, however, could still be detected after 160 min by immunological and chemical assays. Attachment of the P22 phage, which requires O-specific side chains with more than one repeating unit for adsorption, was demonstrated 10 min after the addition of d-galactose. Attachment of the Felix O-1 phage, which requires a complete core, was observed between 20 and 80 min after the addition of d-galactose. The rough specific phages 6SR and Br2 did not adsorb to the bacteria at any time after the addition of d-galactose. By passive hemagglutination inhibition, the presence of O-specific structures could be demonstrated after 10 min. No antigenic activity of the Ra and Rb structures was observed in the LPS preparations isolated at any time after the addition of d-galactose. Methylation analysis of LPS preparations isolated at 10 and 160 min after the addition of d-galactose showed that the O-specific side chains contained an average of 11 and 15 repeating units, respectively. In the 10-min sample, every 25th “Rc structure” carried a side chain, compared to every 3rd residue in the 160-min sample.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKMANN I., LUEDERITZ O., WESTPHAL O. ZUR IMMUNCHEMIE DER SOMATISCHEN ANTIGENE VON ENTEROBACTERIACEAE. IX. SEROLOGISCHE TYPISIERUNG VON SALMONELLA-R-ANTIGENEN. Biochem Z. 1964 May 22;339:401–415. [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Influence of O side chains on the attachment of the Felix O-1 bacteriophage to Salmonella bacteria. J Bacteriol. 1969 Aug;99(2):513–519. doi: 10.1128/jb.99.2.513-519.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Studies of a receptor for felix O-1 phage in Salmonella minnesota. J Gen Microbiol. 1967 Aug;48(2):225–233. doi: 10.1099/00221287-48-2-225. [DOI] [PubMed] [Google Scholar]
- NAIDE Y., NIKAIDO H., MAEKELAE P. H., WILKINSON R. G., STOCKER B. A. SEMIROUGH STRAINS OF SALMONELLA. Proc Natl Acad Sci U S A. 1965 Jan;53:147–153. doi: 10.1073/pnas.53.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. I. Linkage between o side chains and R core. J Biol Chem. 1969 Jun 10;244(11):2835–2845. [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Weiner I. M. Biochemical aspects of structure, differentiation and morphogenesis in microorganisms. Mechanism of biosynthesis of the lipopolysaccharide of Salmonella. Fed Proc. 1967 Jan-Feb;26(1):70–76. [PubMed] [Google Scholar]
- ROBBINS P. W., KELLER J. M., WRIGHT A., BERNSTEIN R. L. ENZYMATIC AND KINETIC STUDIES ON THE MECHANISM OF O-ANTIGEN CONVERSION BY BACTERIOPHAGE EPSILON-15. J Biol Chem. 1965 Jan;240:384–390. [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TINELLI R., STAUB A. M. [Immunochemical study on Salmonella. 7. Study of the products formed by the controlled acid hydrolysis of the polysaccharide extracted from S. typhi. Part 1. Analysis of antigen O: 12 in the Kauffmann-White scheme]. Bull Soc Chim Biol (Paris) 1960;42:583–599. [PubMed] [Google Scholar]
- Wright A., Dankert M., Robbins P. W. Evidence for an intermediate stage in the biosynthesis of the Salmonella O-antigen. Proc Natl Acad Sci U S A. 1965 Jul;54(1):235–241. doi: 10.1073/pnas.54.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]



