Abstract
A recent case-control study implied an inverse correlation between the measured body burden of dioxins (polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans, PCDD/F) and the risk of soft tissue sarcoma in normal population exposed to dioxins mainly via food. The surprising result could not be explained by biases or confounding. There is no a priori confounding by occupational chemicals in a random sample from general population, but exposures to other lipid soluble chemicals with similar sources might be expected to associate with that of dioxins. One such group is polychlorinated biphenyls (PCB). Therefore three most relevant dioxin-like PCB compounds PCB 77, PCB 126, and PCB 169 were now analyzed from the same patients. Cases were 110 soft-tissue sarcoma patients undergoing surgery for their disease, and referents were 227 patients operated for appendicitis. Dioxin and PCB concentrations were analyzed from subcutaneous fat samples by high-resolution gas chromatography–mass spectrometry and TCDD equivalent concentrations (WHO-TEq) were calculated by using toxicity equivalency factors of WHO. The highest risk of sarcoma was found in the septile with the lowest body burden of sum WHO-TEq, and the differences of septiles 2 and 6 from septile 1 were statistically significant. If soft sarcoma risk is true at high occupational levels of dioxins, the provocative result suggests that a possibility of a J-shaped dose-response curve should be taken into consideration and studied further. This is also supported by the similar J-shaped dose responses in animal studies.
Keywords: dioxin, TCDD, PCB, soft tissue sarcoma, individual exposure measurement, body burden, environmental exposure, case-control study, cancer extrapolation, risk assessment
INTRODUCTION
There has been a continuous search for a “physiological” function of AH receptor (dioxin receptor) (Poellinger, 2000). In this discussion the fact that induction of metabolism of foreign substances via AH receptor is a physiological function, has been less often emphasized. A physiological function means implicitly that at some level of activation, the action of AH receptor is useful as a response to environmental stimuli. In fact it would be strange that a highly conserved gene (Hahn, 2002) would have been maintained, unless the function is useful or even a necessity for life.
On the other hand it is clear that dioxin-like compounds cause a plethora of adverse effects at high dose levels (for a review, see Pohjanvirta and Tuomisto, 1994). Most of these are AH receptor-dependent and they include various developmental effects (Abbot and Birnbaum, 1989, 1990, Alaluusua et al., 1993, Kattainen et al., 2001, Mably et al., 1992), thymic atrophy and immunological effects (Kerkvliet et al., 1990), various hyperplastic/metaplastic effects (Poland and Knutson 1982) and cancer (Kociba et al., 1978). In humans several effects have been seen after high accidental or occupational doses, e.g. chloracne and pigmentation (Sweeney and Mocarelli 2000, Geusau et al., 2001), several developmental effects such as growth retardation, hyperpigmentation, neurobehavioural changes, and alterations of sexual development after intrauterine exposure (Rogan et al., 1988, Masuda et al., 1996, Feeley and Brouwer 2000) and tooth deformities after accidental exposure during childhood (Alaluusua et al., 2004). A modest increase in total cancer is suggested by occupational cohort studies (Kogevinas 2000). Increases of gastrointestinal and hematopoietic malignancies have been seen after a TCDD release accident in Seveso, Italy (Bertazzi et al., 1997, 2001). Soft-tissue sarcoma was increased toward the high end of occupational exposures in a large industrial cohort (Fingerhut et al., 1991, Steenland et al., 1999).
Previous cancer studies suffer from poor exposure assessment. In most studies exposure information is based on indirect methods such as work histories, sometimes supported by chemical analyses in part of the studied population and modeling, or in many cases on questionnaire information. Therefore we recently undertook a major attempt of studying soft tissue sarcoma risk in general population in correlation to dioxin concentrations (Tuomisto et al., 2004). Sarcoma patients coming to surgery for their tumor were analyzed for their dioxin concentrations, and in a case-control setting appendicitis patients were studied as referents. A surgical patient control group was needed, because dioxin analysis was from a subcutaneous fat sample. Among the general population in Finland the variation in dioxin concentrations is very large, because the dominant source of dioxins is fish, and fish consumption varies widely in the population. In this study no positive correlation was seen between the dioxin concentrations and soft tissue sarcoma risk, on the contrary, sarcoma risk was highest among those having the lowest dioxin level.
Now three PCB congeners contributing most to the dioxin equivalents have been analyzed in addition to the 17 PCDD/F congeners, and we are able to present data on these congeners added to the total burden of dioxins.
MATERIALS AND METHODS
A detailed description of the methods was given in the previous paper (Tuomisto et al., 2004). Therefore only the most pertinent details are shortly described here.
Study population
Most sarcoma patients in southern Finland are treated by the multidisciplinary sarcoma group of Helsinki University Hospital, and the rest in the University Hospitals of Kuopio, Turku, or Tampere. All patients over 15 years of age referred to these hospitals for operative treatment of STS between June 1997 and December 1999 were eligible as cases. Patients over 15 years of age, operated due to an appendicitis diagnosis in any study hospital were eligible as controls. They were collected from the same catchment area as the STS patients. Southern Finland was divided into 15 areas, and one hospital performing appendectomy operations was recruited to the study from each area (in Helsinki, two hospitals).
Informed consent was obtained from all patients in writing before the operation. The study was duly approved by the Ethics Committees. The total number of patients recruited during the fieldwork was 972, and after exclusion of some patients for technical reasons (see Tuomisto et al., 2004), 954 patients (148 cases and 806 controls) were available for matching.
The cases and controls were individually matched for area and age at the end of the fieldwork. Area was defined as the area of residence of the patient using the 15 areas above. The age was determined at the day of operation. Maximum allowed age difference between cases and controls was ±3 years, if case was <38.0 years old, and ±6 years, if case was >38.0 years old. The control closest by age was matched to the case. Cases with fewer controls had a priority over cases with more controls. The number of controls per case was limited to three. For 110 cases 227 matching controls could be found in the pool. Thirty-nine cases had one control, 25 cases had two, and 46 cases had three controls.
Exposure assessment
A subcutaneous fat sample of the matched 337 patients, obtained during an appendectomy or sarcoma operation, was analyzed for the 17 toxic polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and three dioxin-like non-ortho polychlorinated biphenyls: PCB 77 (3,3′,4,4′-tetrachlorobiphenyl), PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl), and PCB 169 (3,3′,4,4′,5,5′-hexachlorobiphenyl). Measurements were done by gas chromatography – mass spectrometry (Vartiainen et al., 1997) at the Laboratory of Chemistry, which is an accredited testing laboratory (T077) for the analysis of dioxins in human samples (current standard: EN ISO/IEC 17025) and has successfully participated in WHO/Euro intercalibrations. The concentrations were summed up after the value of each congener was multiplied by its relative toxic potency (toxic equivalency factor, TEF). The TEF values according to WHO (Van den Berg et al., 1998) were used, resulting in toxic equivalent concentrations (WHO-TEq) comprising 17 PCDD/Fs and 3 PCBs. All analytical work was performed blind so that the chemistry laboratory did not know the diagnosis of the patient. Strict quality assurance measures were undertaken.
Patients were also asked detailed questionnaire information about socio-economic and lifestyle factors and chemical exposures. Of the matched subjects, 84 cases (76 %) and 185 controls (81 %) have also questionnaire information.
Statistical analyses
Conditional logistic regression analysis was performed with SAS PHREG procedure. Odds ratios were estimated for each septile of WHO-TEq. All analyses were adjusted for sex. Several variables collected with the questionnaire were used as confounders in the analysis one by one. Non-binary variables were analyzed as quartiles.
Exposure to the following chemicals was asked as a binary variable: solvents, solvent-based paints, formaldehyde, insecticides, fungicides/herbicides, wood preservatives, strong detergents, heavy metals, and other chemicals.
RESULTS
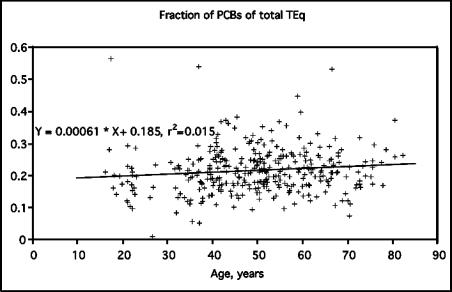
Contribution of PCB-TEqs to total WHO-TEq was similar in all age groups, about 20 % on average (Fig. 1). On the other hand, individual variation was large, and PCB contribution was from 1 % to 57 %. The range of total TEq values was from 4.6 to 197.8 ng/kg (in fat). There was high correlation between PCB-TEq and PCDD/F-TEq (Pearson correlation coefficient 0.84) and between PCB-TEq and total WHO-TEq (r=0.91).
FIGURE 1.
Fractions of PCB-TEqs of the total TEq of PCDD/Fs and PCBs. Each point represents one individual in the study.
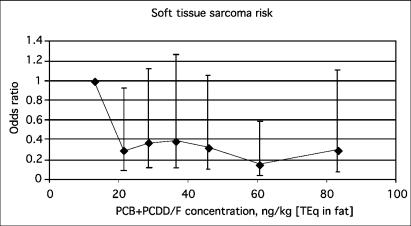
The STS risk was higher in the lowest septile than in other septiles (Fig. 2.), and the difference was significant in the second and the sixth septile. The odds ratios (compared with the lowest septile) varied from 0.15 (95 % CI 0.04 – 0.6) to 0.39 (95 % CI 0.12 – 1.27). When the analysis was performed with WHO-TEq quintiles instead of septiles, the odds ratios were 1, 0.44 (0.18–1.09), 0.73 (0.29–1.84), 0.40 (0.14–1.13), and 0.49 (0.16–1.49). The analysis was also calculated using total WHO-TEq concentration as a continuous linear variable showing a decreasing trend (OR 0.86 for an interquartile TEq increase of 33.32 ng/kg [WHO-TEq in fat], 95 % CI 0.60 – 1.24). When the three PCB-compounds were analyzed separately, the continuous linear variables were: PCB 77, 1.08 (0.93–1.26); PCB 126, 0.92 (0.7–1.22); PCB 169, 0.93 (0.68–1.27), and the sum TEq of all three PCBs 0.92 (0.69–1.23).
FIGURE 2.
Odds ratios (95 % CI) for STS as a function of total TEq concentration in fat (PCDD/F + PCB, presented as the median of each septile). The cases were individually matched for area and age, and sex was controlled as confounder.
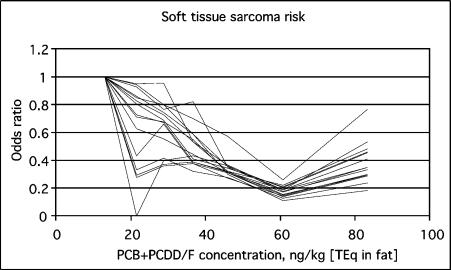
The decrease in odds ratios was not abolished by any of the studied confounders (sex, age, education, body mass index, fish consumption, smoking, alcohol consumption, town size, and chemical exposure) (Fig. 3). On the other hand, including confounders in the analysis tended to cause the odds ratios to decrease in more linear fashion than in the basic result, especially to straighten the kink of the second septile. At the same time, however, the statistical significance of decreased odds ratio disappeared in septile 2 (which was the most unstable septile) in most analyses and in septile 6 in some analyses. A continuous linear analysis including fish as a confounder showed a decrease (OR 0.83 for an interquartile TEq increase, 95 % CI 0.53 – 1.30).
FIGURE 3.
Odds ratios as a function of total TEq concentration (presented as the median of each septile). Each line represents an analysis with a different confounder.
DISCUSSION
The present results indicate that the previous analysis comprising only 17 PCDD/F congeners does not essentially change, when the non-ortho PCB congeners, which contribute most to the PCB-TEq are also included in the sum analysis. The result adds to confidence that previously reported result between dioxin-TEQ and decreased cancer risk of cancer is not confounded by the levels of PCB. In the previous study each dioxin congener was also analyzed separately, and a general trend was that the congeners contributing most to WHO-TEq showed a result similar to the main analysis of sum TEq. In the present study PCB 126 (which contributes most to PCB-Teq), and PCB 169 indicated even alone the same trend of decreasing risk. It should be noted that the concentrations of all these compounds correlate with each other, since their sources are in part similar.
The contemporary body burden is considered a good measure for lifelong exposure to dioxins and PCB compounds. The half-life of TCDD is 7 to 8 years (Poiger and Schlatter, 1986, Pirkle et al., 1989, Flesch-Janys et al., 1996) and this means at a constant intake a cumulation of about 40 years before reaching steady state. Because exposure is mainly from food (Liem et al., 2000), fairly stable exposure can be assumed on an average, and considering the long half-life, the present concentration means integration of exposures during at least 10–20 years, in most cases probably over the whole lifetime. Although different from congener to congener, elimination half-lives of all dioxins and PCBs are very long, and such integration can be assumed to hold for WHO-TEqs as well.
Present exposure in Finland is mainly from Baltic fish, up to over 80% (Kiviranta et al. 2001), although during previous decades also meat and dairy products contributed to some extent (Hallikainen and Vartiainen, 1997). Because fish consumption varies from person to person, this could be assumed to be one of the major differences causing variation in dioxin and PCB levels in different people. An obvious hypothesis then could be that fish consumption might decrease cancer risk, and cause the decreasing trend observed. However, if fish consumption was added to the analysis as a confounder, the main result did not change. Interestingly the analysis improved in the sense that the decrease associated with increasing dioxin TEqs became more regular and linear. These issues require further scrutinizing.
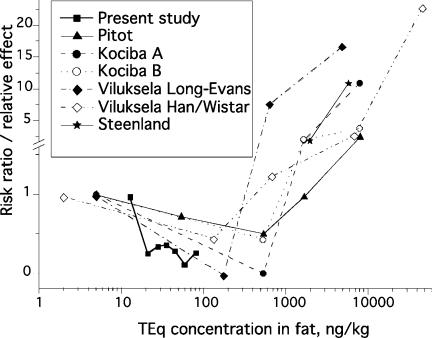
In animal studies, TCDD is a clear carcinogen in several species at high doses (Kociba et al., 1978, Pitot et al., 1987, NTP 1982, Goodman and Sauer, 1992). However, a tendency of less tumors or altered hepatic foci (indicating a carcinogenic process) were seen in animals given low doses of TCDD (ca. 1 ng / kg body weight / day), than were present in the controls in several studies (Kociba et al., 1978, Pitot et al., 1987, Viluksela et al., 2000). This dose would lead to a TCDD concentration in rats of approximately 100 – 500 ng/kg (TCDD in fat) (Kociba et al., 1978, Viluksela et al., 2000). This matches with the higher end of the human concentrations in the present study (Fig. 4).
FIGURE 4.
Comparison of risk ratios from a chronic rat carcinogenicity bioassay (Kociba et al., 1978, A is only hepatocellular carcinomas in female rats, B is hepatocellular carcinomas, hepatocellular hyperplastic nodules, and bile duct adenomas), two tumor promotion assays (Pitot et al., 1987; Viluksela et al., 2000, two rat strains, sensitive Long-Evans and resistant Han/Wistar) and the present study. Abscissa is the measured TCDD (Kociba et al., 1978) or TEq (the present study) concentration in adipose tissue. For the data of Pitot et al. the same concentrations after the same doses were assumed as given by Kociba et al., the lowest dose (0.1 ng/kg/d) was assumed to cause one tenth of that after the lowest dose measured by Kociba et al. (1 ng/kg/d). Also the risk of soft tissue sarcoma given by Steenland et al. (1999) is given at two levels, the whole subcohort of measured values (233 ng/kg fat; Fingerhut et al., 1991) backcalculated at 2000 ng/kg (Steenland et al., 1999), and the high-exposure subgroup (418 ng/kg fat; Fingerhut et al., 1991) approximated by us at 6000 ng/kg. These backcalculations may include a major error, if half-life after a high exposure is much shorter than assumed (Aylward et al., 2005). This error would mean higher actual concentrations at the end of exposure, and move the points to the right even by one order of magnitude or more.
Andersen and Conolly (1998) have suggested a mechanistic model to explain the U-shaped dose-response in liver tumors described by Pitot et al. (1987). The model assumes that some (but not all) of the mutated cells are sensitive to the mitoinhibitory effect of TCDD, resulting in a decrease in the number of mutated cells at low doses. Further studies to scrutinize such mechanisms are urgently needed.
It may also be worthwhile to note that the non-induced activity of some CYP enzymes, notably CYP1A1, is very low (Whitlock 1999). A slight induction could be expected to facilitate the metabolism of many xenobiotics including carcinogenic polycyclic hydrocarbons. Depending on the relative activity of the next stage of metabolism, and possible interactions of dioxins with phase II conjugation reactions (cf. Nguyen et al., 2003), clearance of such compounds could then be increased and their chances of acting as cancer initiators would be decreased.
In conclusion, the previously reported increased risk of STS at the lowest levels of WHO-TEq was maintained when three most important dioxin-like PCBs were included in the analysis. The provocative result within the present population levels of dioxin exposure challenges the present carcinogenicity estimates based on linear extrapolation from high to low doses. The existence of a true J-shape dose-response curve in carcinogenesis by dioxin-like compounds requires more scrutiny, but there are biologically plausible reasons to consider this possibility.
ACKNOWLEDGEMENTS
We thank the surgeons collecting the samples, the personnel in all study hospitals, Mr. Sami Penttinen for managing the study process in hospitals around Finland, and Ms. Seija Nyholm, Ms. Sirkka Markkanen, Ms. Tuula Rissanen, Ms. Katri Mehtonen, Ms. Eija Mehtonen, Mr. Tuomo Korhonen, Ms. Arja Tamminen, and Ms. Minna Voutilainen for technical assistance. The study was funded by the European Commission, Contracts No. ENV-CT96-0336 and QLK4-CT-1999-01446, and by the Academy of Finland, Grant number 43984, 53307, 77298.
Abbreviations
- STS
soft tissue sarcoma
- IARC
International Agency of Research on Cancer
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- PCDD/Fs
polychlorinated dibenzo-p-dioxins and dibenzofurans
- in congener nomenclature:
T=tetra, Pe=penta, Hx=hexa, Hp=hepta, O=octa, CDD=chlorinated dibenzo-p-dioxin, CDF=chlorinated dibenzofuran
- PCBs
polychlorinated biphenyls
- TEF
toxic equivalency factor
- WHO-TEq
toxic equivalencies according to World Health Organization (WHO)
REFERENCES
- Abbott BD, Birnbaum LS. Cellular alterations and enhanced induction of cleft palate after coadministration of retinoic acid and TCDD. Toxicol Appl Pharmacol. 1989;99:287–301. doi: 10.1016/0041-008x(89)90011-2. [DOI] [PubMed] [Google Scholar]
- Abbott BD, Birnbaum LS. Effects of TCDD on embryonic ureteric epithelial EGF receptor expression and cell proliferation. Teratology. 1990;41:71–84. doi: 10.1002/tera.1420410108. [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Lukinmaa P-L, Pohjanvirta R, Unkila M, Tuomisto J. Exposure to 2,3,7,8-tetrachlorodibenzo-para-dioxin leads to defective dentin formation and pulpal perforation in rat incisor tooth. Toxicology. 1993;81:1–13. doi: 10.1016/0300-483x(93)90152-i. [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Calderara P, Gerthoux PM, Lukinmaa P-L, Kovero O, Needham L, et al. Developmental dental aberrations after the dioxin accident in Seveso. Environ Health Perspectives. 2004;112:1313–1318. doi: 10.1289/ehp.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ME, Conolly RB. Mechanistic modeling of rodent liver tumor promotion at low levels of exposure: an example related to dose-response relationship for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Human Exp Toxicol. 1998;17:683–690. doi: 10.1177/096032719801701208. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Brunet RC, Carrier G, Hays SM, Cushing CA, Needham LL, Patterson DG, Gerthoux PM, Brambilla P, Mocarelli P. Concentration-dependent TCDD elimination kinetics in humans: toxicokinetic modeling for moderately to highly exposed adults from Seveso, Italy, and Vienna, Austria, and impact on dose estimates for the NIOSH cohort. J Exposure Anal Environ Epidemiology. 2005;15:51–65. doi: 10.1038/sj.jea.7500370. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, et al. Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident”. Epidemiology. 1997;8:646–652. [PubMed] [Google Scholar]
- Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, et al. Health effects of dioxin exposure: A 20-year mortality study. Am J Epid. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- Feeley M, Brouwer A. Health risks to infants from exposure to PCBs, PCDDs and PCDFs. Food Additives and Contaminants. 2000;17:325–333. doi: 10.1080/026520300283397. [DOI] [PubMed] [Google Scholar]
- Fingerhut M, Halperin WE, Marlow DA, Piacitelli LA, Honchar PA, Sweeney MH, et al. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. New England J Med. 1991;324:212–218. doi: 10.1056/NEJM199101243240402. [DOI] [PubMed] [Google Scholar]
- Flesch-Janys D, Becher H, Gurn P, Jung D, Konietzko J, Manz A, et al. Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. J Toxicol Environ Health. 1996;47:363–378. doi: 10.1080/009841096161708. [DOI] [PubMed] [Google Scholar]
- Geusau A, Abraham K, Geissler K, Sator MO, Stingl G, Tschachler E. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: Clinical and laboratory effects. Environ Health Perspectives. 2001;109:865–869. doi: 10.1289/ehp.01109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Sauer R. Hepatoxicity and carcinogenicity in female Sprague-Dawley rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): a pathology working group re-evaluation. Regul Toxicol Pharmacol. 1992;15:245–252. doi: 10.1016/0273-2300(92)90036-9. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chemico-Biological Interactions. 2002;141:131–161. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hallikainen A, Vartiainen T. Food control surveys of polychlorinated dibenzo-p-dioxins and dibenzofurans and intake estimates. Food Additives and Contaminants. 1997;14:355–366. doi: 10.1080/02652039709374538. [DOI] [PubMed] [Google Scholar]
- Kattainen H, Tuukkanen J, Simanainen U, Tuomisto JT, Kovero O, Lukinmaa P-L, et al. In utero / lactational TCDD exposure impairs molar tooth development in rats. Toxicol Appl Pharmacol. 2001;174:216–224. doi: 10.1006/taap.2001.9216. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI, Baecher-Steppan L, Smith BB, Youngberg JA, Henderson MC, Buhler DR. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationship and effects in C57B1/6 mice congenic at the Ah locus. Fundam Appl Toxicol. 1990;14:532–541. doi: 10.1016/0272-0590(90)90257-k. [DOI] [PubMed] [Google Scholar]
- Kiviranta H, Hallikainen A, Ovaskainen M-L, Kumpulainen J, Vartiainen T. Dietary intakes of polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in Finland. Food Additives and Contaminants. 2001;18:945–953. doi: 10.1080/02652030110057134. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Keyes DG, Beyer J, Carreon R, Wade C, Dittenber D, et al. Results of the two year toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Kogevinas M. Studies of cancer in humans. Food Additives and Contaminants. 2000;17:317–324. doi: 10.1080/026520300283388. [DOI] [PubMed] [Google Scholar]
- Liem AKD, Fürst P, Rappe C. Exposure of populations to dioxins and related compounds. Food Additives and Contaminants. 2000;17:241–259. doi: 10.1080/026520300283324. [DOI] [PubMed] [Google Scholar]
- Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In Utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1992;114:118–126. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- Masuda Y. Kuratsune M, Yoshimura H, Hori Y, Okumura O, Masuda Y, editors. Causal agents of Yusho. A human disaster caused by PCBs and related compounds. 1996:47–80. [Google Scholar]
- National Toxicology Program. Bioassay of 2,3,7,8-tetrachlorodibenzo-p-dioxin for possible carcinogenicity (oral gavage study). 209, NTP 80-31. 1982. NIH-Publications 82-1765.
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Wolfe WH, Patterson DG, Needham LL, Michalek JE, Miner JC, et al. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of operation Ranch Hand. J Toxicol Environ Health. 1989;27:165–171. doi: 10.1080/15287398909531288. [DOI] [PubMed] [Google Scholar]
- Pitot HC, Goldsworthy TL, Moran S, Kennan W, Glauert HP, Maronpot RR, et al. A method to quantitate the relative initiating and promoting potencies of hepatocarcinogenic agents in their dose-response relationship to altered hepatic foci. Carcinogenesis. 1987;8:1491–1499. doi: 10.1093/carcin/8.10.1491. [DOI] [PubMed] [Google Scholar]
- Poellinger L. Mechanistic aspects - the dioxin (aryl hydrocarbon) receptor. Food Additives and Contaminants. 2000;17:261–266. doi: 10.1080/026520300283333. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in laboratory animals: Effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Poiger H, Schlatter C. Pharmacokinetics of 2,3,7,8-TCDD in man. Chemosphere. 1986;15:1489–1494. [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, Hung KL, Kloong SL, Shih LY, Taylor JS, et al. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science. 1988;241:334–336. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, hearth disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst. 1999;91:779–786. doi: 10.1093/jnci/91.9.779. [DOI] [PubMed] [Google Scholar]
- Sweeney MH, Mocarelli P. Human health effects after exposure to 2,3,7,8-TCDD. Food Additives and Contaminants. 2000;17:303–316. doi: 10.1080/026520300283379. [DOI] [PubMed] [Google Scholar]
- Tuomisto JT, Pekkanen J, Kiviranta H, Tukiainen E, Vartiainen T, Tuomisto J. Soft tissue sarcoma and dioxins - A case control study. Int J Cancer. 2004;108:893–900. doi: 10.1002/ijc.11635. [DOI] [PubMed] [Google Scholar]
- van den Berg M, Birnbaum LS, Boseveld ATC, Brunström B, Cook PH, Feeley M, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspectives. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen T, Saarikoski S, Jaakkola J, Tuomisto J. PCDD, PCDF and PCB concentrations in human milk from two areas of Finland. Chemosphere. 1997;34:2571–2583. doi: 10.1016/s0045-6535(97)00100-8. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Bager Y, Tuomisto JT, Scheu G, Unkila M, Pohjanvirta R, et al. Liver tumor-promoting activity of 2,3,7,8.tetrachlodibenzo-p-dioxin (TCDD) in TCDD-sensitive and TCDD-resistant rat strains. Cancer Res. 2000;60:6911–6920. [PubMed] [Google Scholar]
- Whitlock JP., Jr. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]