Abstract
A variety of cellular activities, including metabolism, growth, and death, are regulated and modulated by the redox status of the environment. A biphasic effect has been demonstrated on cellular proliferation with reactive oxygen species (ROS)—especially hydrogen peroxide and superoxide—in which low levels (usually submicromolar concentrations) induce growth but higher concentrations (usually >10–30 micromolar) induce apoptosis or necrosis. This phenomenon has been demonstrated for primary, immortalized and transformed cell types. However, the mechanism of the proliferative response to low levels of ROS is not well understood. Much of the work examining the signal transduction by ROS, including H2O2, has been performed using doses in the lethal range. Although use of higher ROS doses have allowed the identification of important signal transduction pathways, these pathways may be activated by cells only in association with ROS-induced apoptosis and necrosis, and may not utilize the same pathways activated by lower doses of ROS associated with increased cell growth. Recent data has shown that low levels of exogenous H2O2 up-regulate intracellular glutathione and activate the DNA binding activity toward antioxidant response element. The modulation of the cellular redox environment, through the regulation of cellular glutathione levels, may be a part of the hormetic effect shown by ROS on cell growth.
Keywords: oxidative stress, antioxidant response element, MAPK, biphasic response, signal transduction
INTRODUCTION
The evolution of mammalian cells in the oxidative atmosphere has resulted in development of sensitivity of many cellular functions to the oxidative/reductive status of both the extracellular and intracellular environments. The redox status of the intracellular milleu influences cellular activities including signal transduction, metabolism, growth, apoptosis and cellular systems involved in detoxification. Reactive oxygen species (ROS) including superoxide (O2−·), hydrogen peroxide (H2O2) and hydroxyl radical (HO·) are known to inhibit activities of various biological molecules. More recently, low levels of ROS have been recognized to serve as second messengers for signal transduction (Halliwell, 1996; Suzuki et al., 1997).
O2−· is generated via electron reduction of molecular oxygen O2. Produced O2−· often undergoes disproportionation reactions in which one molecule of O2−· donates an electron to another, forming H2O2 and O2 in a reaction named dismutation. The rate of dismutation reaction can be substantially increased by the enzyme superoxide dismutase (SOD), which occurs in either the copper/zinc (Cu/ZnSOD) or manganese (MnSOD) isoform. In addition to donating an electron to another O2−·, O2−· can also donate an electron to ferric ion (Fe3+) to form ferrous ion (Fe2+), which in turn reduces H2O2 and causes an homolytic fission of the oxygen-oxygen bond to form a very potent oxidant HO· in the Fenton reaction. HO· in turn can react with virtually any orgainic compounds with very high kinetic rate constants, thus damaging a variety of biological molecules. To minimize the production of HO·, H2O2 levels are tightly controlled by several enzymes, including catalase, glutathione peroxidase, thioredoxin and peroxiredoxins (Suzuki et al., 1997; Veal et al., 2004; Wood et al., 2003; Zhao and Holmgren., 2002). Glutathione peroxidase utilizes reduced glutathione (GSH) to perform two electron reduction of H2O2 to produce H2O. Oxidized glutathione can then be reduced via glutathione reductase.
Endogenous generation and exogenous application of ROS have been shown to directly activate specific signaling pathways. Cellular responses to large changes in the redox state of the environment are designed to maintain the cellular oxidative balance and avoid DNA, protein and lipid damage which could potentially lead to apoptosis or necrosis. However, in some cases, the cellular response to small changes in the ROS concentrations can have beneficial effects on cell growth and viability. This article summarizes work by our laboratory and others on the mechanisms of non-linear actions of ROS on cell growth.
EFFECTS OF ROS ON GROWTH OF PRIMARY CULTURE, IMMORTALIZED AND TRANSFORMED CELLS
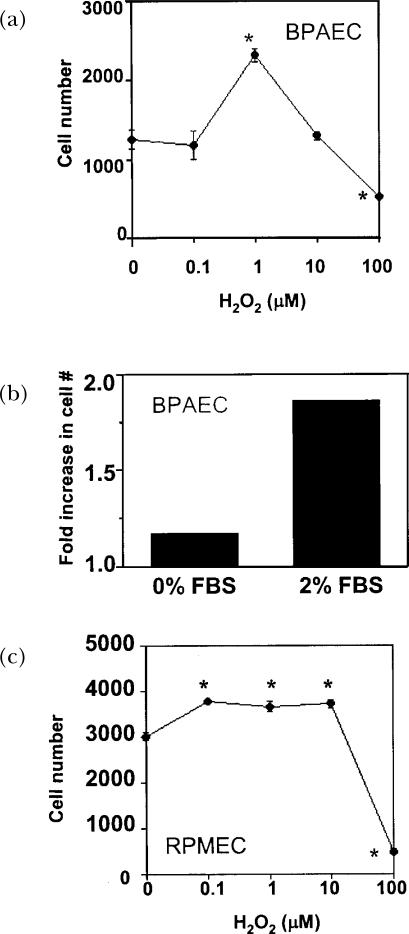
Burdon et al. (1989) demonstrated that low concentrations (10−6 to 10−8 M) of H2O2 and t-butyl hydroperoxide could stimulate growth in both primary fibroblast cultures and transformed baby hamster kidney fibroblasts (BHK-21) in the absence of serum (Burdon et al., 1989). Higher concentration of these ROS caused reduced cell number, while even lower concentrations had no effect on cell numbers, thus delineating a biphasic effect of the compounds (Burdon et al., 1989; Burdon and Rice-Evans, 1989). Later studies showed that the biphasic effect of H2O2 on cell growth also occurred in cultures of untransformed and Ras-transformed fibroblasts, hepatoma cells, cultured smooth muscle cells, primary culture of pulmonary artery endothelial cells (PAEC), primary culture of rat microvascular endothelial cells (PMEC), and immortalized lung epithelial cells (A549) (Arnold et al., 2001; Burdon, 1995; Burdon et al., 1990; Day et al., 2003; Gallagher et al., 1993; Liu et al., 2002). Dose-response H2O2 treatment of primary bovine PAEC (Fig 1A) and primary rat PMEC (Fig 1C) showed the existence of a biphasic curve, where 1 μM H2O2 increased cell number and higher levels (>10 μM) decreased cell number. Although treatment of PAEC with H2O2 alone induced an increase in cell number, the effect of low concentrations of H2O2 was greater in the presence of 2% FBS (Fig 1B) (Day et al., 2003). The exact dosages of H2O2 resulting in cell growth is cell-type and cell density specific; some mammalian fibroblasts display growth in response to H2O2 at levels as high as 15 μM (Davies, 1999), but a biphasic effect is still observed.
FIGURE 1.
Effects of H2O2 on bovine PAEC and RPMEC growth. (A) Bovine PAEC (BPAEC) were treated with varied concentrations of H2O2 for 48 h in medium containing 2% FBS. (B) BPAEC were treated with 1 μM H2O2 for 48 h in medium containing 0 or 2% FBS. (C) RPMEC were treated with varied concentrations of H2O2 for 48 h in medium containing 2% FBS. The number of cells was determined by Coulter counting. Values represent means ± SE, n=3. ∗Values differ from the 0 μM H2O2 control at p<0.05. Work of Day et al. is being reproduced with permission (Day et al., 2003).
O2−· has also been shown at low doses to induce growth in a variety of cell types including human and murine fibroblasts, human leukemia cells, human amnion cells, epithelial cells, and human smooth muscle cells (Burdon, 1995; Li et al., 1997; Li et al., 1999; Murrell et al., 1990). Superoxide generated in these cell cultures using hypoxanthine with xanthine oxidase (≤1 μ unit xanthine oxidase/ml) induced proliferation that was suppressed by co-incubation of cells with superoxide dismutase (SOD) but not by co-incubation with catalase, showing that the growth-inducing species was in fact O2−· and not due to its reduction to H2O2 (Murrell et al., 1990).
MULTIPLE OUTCOMES OF HIGH LEVELS OF ROS TREATMENT IN CULTURED CELLS
Investigation of the effects of higher levels of ROS on eukaryotic cells show the occurrence of temporary growth arrest, senescence, apoptosis, or necrosis (reviews, Boonstra and Post, 2004; Davies, 1999; Fiers et al., 1999; Frippiat et al., 2002; Remacle et al., 1992, 1995). Treatment of fibroblasts with H2O2 showed that growth occurred between 3 to 15 μM. Temporary growth arrest occurred between 120 to 150 μM, associated with the expression of DNA repair and antioxidant enzymes. Permanent growth arrest, not associated with cell death, occurred between 250 to 400 μM. At treatments between 0.5 to 1 mM classical markers for apoptosis were observed, and necrosis occurred at levels above 5 mM (Davies, 1999). FACS and propidium iodide staining were used to show that concentrations of H2O2 from 30 μM–100 μM were associated with apoptotic cell death in primary PAEC; the lower level of H2O2 treatment resulting in apoptosis suggests that these cells were more sensitive than fibroblasts to oxidative stress (Day et al., 2003). In the case of exposure of a macrophage cell line to nitric oxide donors, both apoptosis and necrosis were found; cellular apoptosis was attributed to the generation of H2O2 and caspase activation, while necrosis was attributed to reduced ATP generation and energy failure in conjunction with thiol depletion (Borutaite and Brown, 2003). The differentiation between the outcomes involving cellular senescence, apoptosis and necrosis appears to be cell-type and cell density specific, ROS species specific, and likely involves differential signal transduction and enzyme activation as well as differences in gene expression and/or DNA damage.
WHAT CONCENTRATIONS OF ROS ARE BIOLOGICALLY RELEVANT?
Production of ROS was initially investigated in neutrophils, which generate large quantities of ROS for the destruction of bacteria, infected cells and abnormal cells. However, the production of lower levels of ROS has also been examined in non-leukocytes, and has been demonstrated to occur to varying degrees and in response to a variety of stimuli in all cell types examined to date. The mechanism of generation of ROS by nonphagocytic cell types involves the assembly of NADH or NAD(P)H oxidases at the cell membrane (Griendling and Harrison, 1999; Thannickal et al., 2000). The phagocytic NAD(P)H oxidase has been the most studied, but oxidase complexes containing gp91 homologs (NOX-1, NOX-3 or NOX-4) have been identified in other cell types, including endothelial and epithelial cells, fibroblasts, osteoclasts and vascular smooth muscle cells (Babior, 2000; Bayraktutan et al., 2000; Chamseddine and Miller, 2003; Murphy et al., 2000; Perner et al., 2003; Yang et al., 2001b; Zafari et al., 1998). These NADH and NAD(P)H oxidase systems have been shown to contain a gp91 homolog (NOX), p67 phox, p22 phox and p47 phox (Hohler et al., 2000; Jones et al., 1996; Parinandi et al., 2003; Sorescu and Griendling, 2002; Zafari et al., 1998). Several of the Ras-related small GTP-binding proteins, including Ras and Rac1, have also been implicated in the generation of ROS by the nonphagocytic oxidases (Joneson and Bar-Sagi, 1998; Sundaresan et al., 1996; Thannickal et al., 2000).
Measurements of endogenous generation of active oxygen species have been performed on primary, immortalized and transformed eukaryotic cells. In 1991, Kinnula et al. examined the production of extracellular H2O2 by freshly isolated alveolar epithelial Type II cells, unstimulated alveolar macrophages and primary endothelial cells (Kinnula et al., 1991). Results showed that macrophages produced the highest level of extracellular peroxide (3.1±0.09 nmol·min−1·mg protein−1, equivalent to ∼0.1×106 cells), followed by Type II alveolar cells (0.7±0.07 nmol·min−1·mg protein−1, ∼0.5×106 cells) and then by vascular endothelial cells (0.4 to 0.06±0.005 nmol·min−1·mg protein−1, ∼3×106 cells) (Kinnula et al., 1991; Zulueta et al., 1995). Extracellular superoxide production has also been measured in non-leukocytes. From data available, fibroblasts appear to release the highest levels of O2−· (∼1 nmol·h−1·1×105 cells) (Murrell et al., 1990), followed by endothelial cells (∼0.6 nmol·h−1·1×105 cells) (Weening et al., 1975), and then unstimulated granulocytes (∼0.4 nmol·h−1·1×105 cells) (Matsubara and Ziff, 1986).
The level of ROS produced by cells can be regulated by the redox state of the environment. Exposure of cells to hypoxia increased the release of H2O2 by about 25–30% (Kinnula et al., 1991; Parinandi et al., 2003). Experiments by Parinandi et al. (2003) showed that exposure of PAECs to hyperoxia induced ∼2.5-fold increase in ROS production within 1 h (Parinandi et al., 2003). These studies suggest that cells adjust their endogenous ROS production in response to oxidative stress.
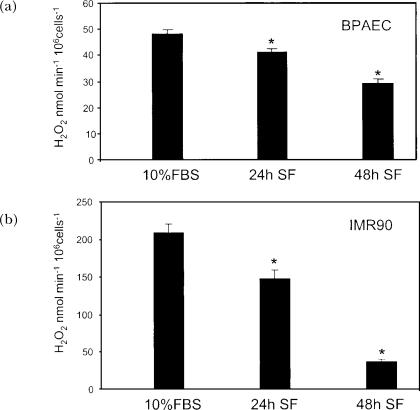
The observations of cellular generation of ROS, and the speculation that these molecules act as second messengers, led to the hypothesis that there is a balance between cell growth and endogenous ROS production (Burdon, 1995). Burdon et al. (1995) examined endogenous intracellular H2O2 production by immortalized BHK-21 fibroblast cells, and found that levels of H2O2 production decreased with increasing time in culture and confluence (from ∼0.15 nmol/106 cells to ∼0.015 nmol/106 cells after 72 h in culture) (Burdon et al., 1995). Sub-confluent transformed epithelial cells also produced significantly more ROS than confluent cells (Perner et al., 2003). Our laboratory has shown that the level of extracellularly generated H2O2 production by primary PAEC and primary human embryonic fibroblasts (IMR90) also decreases with incubation in serum-free medium (Fig. 2). These results show that decreased ROS production correlates with decreased rates of growth.
FIGURE 2.
Effect of growth arrest on endogenous production of extracellular H2O2 by bovine PAEC and IMR90. Bovine PAEC (BPAEC) (A) or IMR90 human lung fibroblasts (B) were subcultured at 4×104 cells/35 mm dish in RPMI supplemented with 10% FBS. 24 h and 48 h after plating, medium was replaced with serum free (SF) RPMI. Extracellular H2O2 was determined as described previously (Thannickal et al., 1993), and normalized to cell numbers. Briefly, cells were washed with Hanks' buffered saline, without phenol red, pH 7.4. Cells were then placed in Hanks saline containing 1mM HEPES, 100 μM homovanillic acid, 5 units/ml horseradish peroxidase, type IV. The conditioned medium was collected after 1 h, and the pH was adjusted to 10.0 with 0.1 M glycine-NaOH buffer. Fluorescence was measured at excitation and emission wavelengths of 321nm and 421nm, respectively. Control samples were made containing the reaction mixture alone, and incubated in the absence of cells to correct for spontaneous dimerization of homovanillic acid. A standard curve was generated using known concentrations of H2O2 incubated with the reaction solution. Values represent means ± SD, n=3. ∗Values differ from 10% FBS at p<0.05.
Interestingly, some tumor cell lines constitutively produce high levels of extracellular ROS, which are associated with their increased proliferation (Perner et al., 2003; Pustovidko et al., 2000; Schimmel and Bauer, 2002; Suh et al., 1999). Exogenous expression of NOX1 in NIH3T3 fibroblasts caused increased cell growth, abnormal cellular morphology and the ability to form tumors in athymic mice (Arnold et al., 2001). In these experiments, 10-fold over-expression of NOX-1 in NIH3T3 fibroblasts induced increased growth and transformation with only <2-fold increase in extracellular O2−· generation, showing that high levels of ROS are not required to achieve a growth or transformation. Co-expression of catalase reversed the transformed phenotype, indicating that, in this case, H2O2 was the growth-promoting species (Arnold et al., 2001).
SIGNAL TRANSDUCTION BY HIGH VERSUS LOW DOSES OF ROS
Studies of ROS-induced signal transduction have mostly focused on the use of large doses of ROS and their cellular responses. These studies have been instrumental in the identification of signaling pathways and specific molecules responsive to ROS. Exogenously added ROS at high levels (>10 μM) directly inactivate protein phosphatases, thus shifting the balance of kinase/phosphatase activity in the cell toward increased phosphorylation events, and resulting in the activation of specific signaling pathways (Guy et al., 1993; Rhee et al., 2000; Suzuki et al., 1997; Thannickal and Fanburg, 2000). Additionally, ROS are believed to directly activate some kinases, including protein kinase C and MAP kinase (Gopalakrishna and Anderson, 1989; Suzuki et al., 1997; Thannickal and Fanburg, 2000). Recently, it was shown that the heterotrimeric proteins Gi and Go can also be directly activated by ROS (Nishida et al., 2002).
Low oxygen environments induce both the stability and activity of the hypoxia-inducible factors (HIFs) which regulate transcription of a variety of growth factors and glycolytic enzymes for anaerobic energy production (Hoshikawa et al., 2003; Lando et al., 2002). Highly oxidative environments also result in the induction of detoxification enzymes, via transcription factors including the antioxidant response element (ARE) binding proteins (such as members of the Nrf family), activator protein 1 (AP-1), and NF-κB (Griffith, 1999; Thannickal and Fanburg, 2000; Wild and Mulcahy, 2000).
In contrast with the numbers of studies performed using toxic levels of ROS (usually >30 μM), relatively few studies have been performed to examine signal transduction by lower concentrations of ROS. As stated above, 10-fold over-expression of NOX-1 in NIH3T3 fibroblasts induced cellular growth and transformation with <2-fold increase in extracellular O2−· generation; growth was associated with increased expression of a variety of genes related to cell cycle regulation and cancer but not to oxidative stress (Arnold et al., 2001). A study by Cumming, et al., examined global changes in disulfide bond formation following ROS exposure (Cumming et al., 2004). The results showed treatment of HT22 cells to 10 μM H2O2 affected several lower molecular weight proteins but had little effect on the disulfide bonding of proteins in the 40–150-kDa range. However, a number of disulfide bonded proteins in the 40–150-kDa range were affected by 150 or 400 μM H2O2 treatment. The results suggest that uniquely disulfide bonded proteins which occur at low levels of H2O2 exposure may be involved in the mitogenic effects of low oxidant concentrations on cultured cells. In contrast, proteins that are disulfide-linked at intermediate H2O2 concentrations may participate in the adaptive response or growth arrest. These findings support the hypothesis that growth-inducing levels of ROS may not signal via the same pathways as those found to be activated by treating cells with high doses of ROS associated with growth arrest or apoptosis.
Extracellular ligands including fibroblast growth factor, platelet-derived growth factor, endothelin, interferon γ, tumor necrosis factor α, and members of the interleukin family have been shown to induce the production of low levels of intracellular ROS (Finkel, 2000; Finkel, 2001; Thannickal and Fanburg, 2000). Antioxidants or antioxidant enzymes have been used to inhibit signal transduction downstream of these factors as a method of identifying specific proteins responsive to ROS second messengers. Such experiments have been used to prove ROS inactivation of protein phosphatases, as well as activation of signal transduction proteins such as MAP kinases (including p38, p42/p44 and jun kinase), phospholipase A2, and the transcription factors NF-κB, Egr-1 and c-Fos (Finkel, 2001; Thannickal and Fanburg, 2000; Xu et al., 2002). In many cases the elimination of ROS generation abrogates downstream biological responses, including cell growth (Xu et al., 2002).
Changes in the redox environment can also be attained by over-expression or inhibition of anti-oxidant enzymes, with subsequent effects on cell growth/apoptosis and signaling. Adenoviral over-expression of catalase (50–100-fold excess) causes decreased proliferation and DNA synthesis and a rise in apoptosis in vascular smooth muscle cells (Brown et al., 1999). Vector expression of Cu/Zn-SOD in microglial cells prevents cellular activation by LPS; here, the inhibition is hypothesized to occur due to scavenging of O2−·, a second messenger required for LPS signaling (Chang et al., 2001).
As discussed above, many cancer cell lines produce increased levels of ROS; some of these transformed cells also express higher levels of SOD and/or catalase, possibly to catalyze the breakdown of excess superoxide and H2O2 (Chung-man Ho et al., 2001; Grigolo et al., 1998; Hur et al., 2003; Janssen et al., 1999; Malafa et al., 2000; Palazzotti et al., 1999; Suresh et al., 2003). This adaptive effect protects the cells from the higher level of oxidant stress, and also often results in their resistance to anti-cancer treatments (Chung-man Ho et al., 2001; Hur et al., 2003; Suresh et al., 2003; Yang et al., 2001a). Even in these cases, with up-regulation of both ROS production and antioxidant enzymes, the overall redox balance is maintained to favor growth-promoting signal transduction by the ROS. In ovarian cancer cells, pancreatic adenocarcinoma and prostate tumor cells, exogenous over-expression of MnSOD led to reduced growth rates (Cullen et al., 2003; Plymate et al., 2003; Takada et al., 2002). In some cases, scavenging of H2O2 inhibited the proliferation of transformed fibroblasts and reverted their phenotype (Arnold et al., 2001; Preston et al., 2001).
GLUTATHIONE AND ANTIOXIDANT ENZYME LEVELS: REDOX BALANCE FOR GROWTH, QUIESCENCE OR APOPTOSIS
The primary defenses of eukaryotic cells against oxidation include antioxidant enzymes SOD and catalase, and GSH-utilizing mechanisms. GSH, which is present in millimolar concentrations in cells, acts to prevent the oxidation of other cellular molecules and functions in Phase II detoxification of xenobiotics (Griffith, 1999; Wild and Mulcahy, 2000). The ratio of intracellular thiol reductants and ROS appears to play a pivotal role in determining whether cells undergo growth or apoptosis (Burdon, 1995). Imbalance of the redox status, either by high levels of oxidation or increase of reducing agents, favors decreased growth and increased apoptosis.
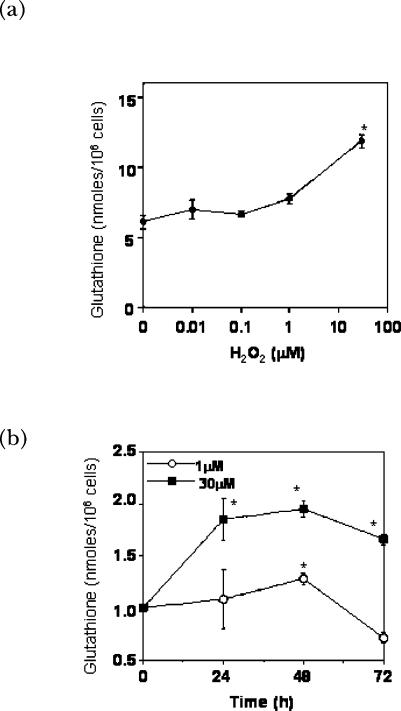
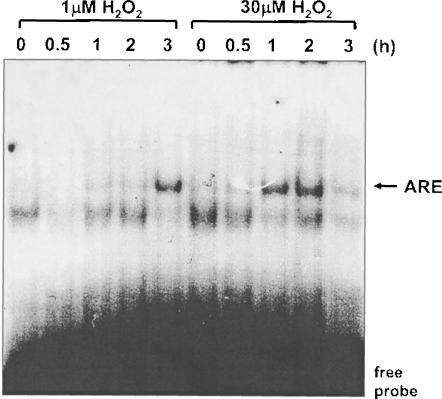
The level of cellular GSH is responsive to the redox state of the cellular environment as well as the growth state of the cell (Burdon et al., 1994; Day et al., 2003; Griffith, 1999; Wild and Mulcahy, 2000). GSH is increased in response to endogenous and exogenously added active oxygen species (Burdon et al., 1994; Day et al., 2003; Day et al., 2002). The direct response of cells to an increase in the oxidation state of the environment occurs through the transcriptional regulation of genes that regulate glutathione synthesis as well as uptake of GSH precursors (Day et al., 2003; Day et al., 2002; Deneke and Fanburg, 1989; Deneke et al., 1987). Our laboratory has shown that sub-lethal doses of H2O2, like toxic levels of H2O2, up-regulate cellular glutathione levels although the time course is delayed by ∼24 h (Fig. 3) (Day et al., 2003). Although 1 μM H2O2 does not activate p42/p44 MAP kinase or NF-κB in PAEC, this low concentration of H2O2 does activate the binding of other transcription factors to the antioxidant response element (ARE), such as Nrf2, as determined by band shift assays (Fig. 4) (Day et al., 2002; Day et al. 2003) and by Nrf2 expression (Pi et al., 2003). Interestingly, there is a 2 h delay in the activation of ARE-binding proteins by 1 μM compared with the 30 μM dose. The ARE element in the promoter of γ-glutamylcysteine synthetase (γGCS) has been shown to be required for the regulation of this gene in response to some xenobiotic agents (Wild and Mulcahy, 2000). Because γGCS controls a key step in the synthesis of glutathione, regulation of this enzyme is of interest for understanding cellular response to ROS.
FIGURE 3.
Effects of H2O2 on cellular glutathione content. (A) Bovine PAEC (BPAEC) were treated with varied concentrations of H2O2 for 48 h in medium containing 2% FBS. Levels of total glutathione were determined as previously described (Day et al., 2003). Briefly, cells were detached from dishes using trypsin/EDTA to produce a cell suspension. A portion of the suspension was used to obtain a cell count while the remaining cells were treated with 1% perchloric acid. The perchloric acid supernatants were sonicated on ice and adjusted to pH 7. Total glutathione was measured in a kinetic assay using glutathione reductase, β-NADPH and gluthathione disulfide. The reduction of 5,5′-dithiobis(2-nitrobenzoic acid) was followed spectrophotometrically at 412 nm. Values represent means ± SD, n=3; experiments were performed at least 3 times. ∗Values differ from 0 μM H2O2 at p<0.05. (B) Bovine PAEC were treated with 1 or 30 μM H2O2 for the durations indicated. The graph shows means ± SE, n=3, of fold increase in glutathione. ∗Values differ from 0 time point at p<0.05. Work is being reproduced with permission (Day et al., 2003).
FIGURE 4.
Effects of H2O2 on ARE binding. Bovine PAEC were treated with the indicated concentrations of H2O2 for 0.5 to 3 h. Nuclear extracts were prepared and DNA-binding activity to an ARE consensus oligo was monitored by EMSA. To prepare nuclear extracts, cells were washed in PBS and incubated in 10 mM Hepes (pH 7.8), 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM NaF, 0.1 mM sodium orthovanadate, and 1 mM tetrasodiumphyrphosphate for 15 min at 4°C. IGEPAL CA-630 was then added at a final concentration of 0.6% (v/v). Samples centrifuged and pelleted nuclei were resuspended in extraction buffer (50 mM Hepes (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 0.1 mM phenyl-methylsulfonyl fluoride, 5 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM NaF, 0.1 mM sodium orthovanadate, and 1 mM tetrasodiumphyrphosphate, and 1% glycerol) and mixed vigorously for 20 min, 4°C, and centrifuged for 5 min. Supernatants were harvested and protein concentrations were determined. To perform EMSA binding reaction, mixtures containing 2 μg nuclear extract protein were incubated with 1 μg poly(dI-dC)·poly(dI-dC) and 32P-labeled double-stranded oligonucleotide containing a consensus sequence for ARE in 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol (v/v), and 20 mM Tris-HCl (pH 7.5) for 20 min at 25°C. Electrophoresis of samples through a native 6% polyacrylramide gel was followed by autoradiography. This work is being reproduced with permission (Day et al., 2003). The primary species binding to the ARE in PAEC was previously identified to be Nrf-2 (Day et al., 2002).
Glutathione imbalances in the cell have been linked to specific changes in signal transduction downstream of growth factors or cytokines, ultimately leading to changes in the overall outcome of signaling. N-acetyl-L-cysteine (NAC, a glutathione precursor which increases intracellular levels of glutathione), inhibits growth induced by endothelin-1 in smooth muscle cells (Kyaw et al., 2002); this affect was attributed to the reduction of second-messenger ROS generation by endothelin-1, leading to the failure to activate specific MAP kinases. In lymphocytes, drastically decreasing cytoplasmic levels of glutathione by treatment with buthiomine sulfoximine causes increased tyrosine phosphorylation in response to tumor necrosis factor α (Anderson et al., 1994; Staal et al., 1994). Levels of oxidized/reduced glutathione may also play a direct role in signal transduction, especially with regard to Ca2+ release from intra-cellular stores and cellular response to oxidative stress (Marcho et al., 1991; Suzuki et al., 1998). In many cases, an increased oxidative environment appears to favor increased kinase activity, while an increased reductive environment often correlates with suppressed kinase activation (Guy et al., 1993; Suzuki et al., 1997), although the overall balance remains critical for the proliferative outcome.
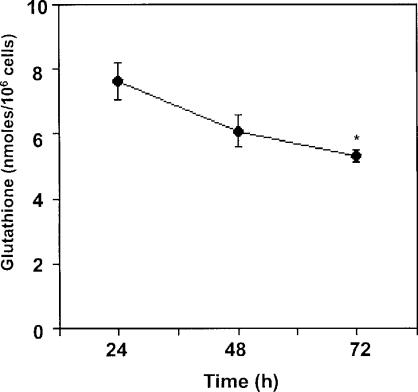
Experiments examining glutathione levels in growing versus growth arrested cells show that glutathione levels become reduced as cells approach quiescence (Fig. 5) (Burdon et al., 1994; Day et al., 2003); the reduction of endogenous GSH production occurs at the same time that cells are producing reduced levels of extracellular ROS, again suggesting that a cellular balance is being maintained (compare Fig. 2). Endothelial cell growth inhibition by treatment with transforming growth factor beta1 (TGFβ1) is associated with both decreased cellular glutathione and increased extracellular production of H2O2 (Das et al., 1992; Thannickal et al., 1993; White et al., 1999). TGFβ-associated antiproliferative effects were found to be modulated by simultaneous treatment of PAEC with cysteine, cystine or NAC, precursors of glutathione synthesis (Das et al., 1992; White et al., 1999), again demonstrating that a balance between ROS and GSH can determine biological outcome of oxidative stress.
FIGURE 5.
Effects of cell density on glutathione content. Bovine PAEC were grown for the durations indicating after plating at 4×104 cells/35 mm dish in RPMI supplemented with 10% FBS. Total cellular glutathione was measured as stated in Figure 3. Values represent means ± SE, n=3. ∗Values differ from the 24 h value at p<0.05. Work is being reproduced with permission (Day et al., 2003).
CONCLUSIONS
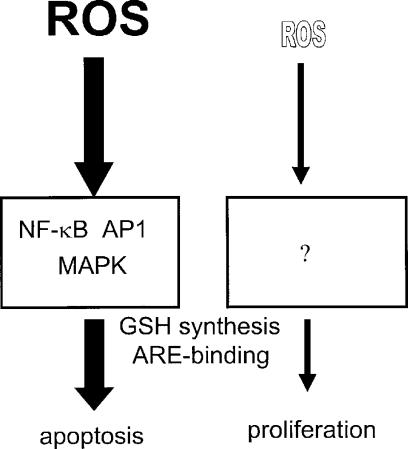
The response of eukaryotic cells to active oxygen species can induce proliferation, growth arrest, apoptosis, or necrosis, depending upon the dose of ROS exposure. Our results show that in PAEC, 0.1 to 1.0 μM H2O2 causes growth, ∼30 to 100 μM induces apoptosis, while higher levels may induce necrosis (Fig 6). The outcome of exposure to oxidative stress can vary with cell density and is cell type-specific, with some cell types showing a higher tolerance toward the apoptotic effects. The biphasic effect of ROS treatment on eukaryotic cells has been hypothesized to arise from the potential disruption in the balance in the redox environment of the intracellular milieu. Oxidative species function as second messenger molecules downstream of a number of growth-promoting factors, but ROS are also highly reactive and can cause damage to cellular proteins, lipids and DNA. Therefore, a delicate balance exists between the useful function of these molecules versus their destructive nature. While a number of signal transduction pathways have been identified for high doses of ROS in cells, very little is known about signal transduction by low, growth promoting levels of ROS. High dose ROS signaling is likely related to cellular apoptosis or necrosis, suggesting that these pathways may not be relevant for understanding the mechanisms involved for the proliferative response of cells to micromolar and submicromolar levels of ROS.
FIGURE 6.
Scheme of biphasic ROS signal transduction. High levels of ROS (left side) induce activation of multiple MAP kinases and activate transcription factors including AP-1 and NF-κB; these pathways lead to apoptosis or necrosis. Low doses of ROS (right side) cause cell proliferation, but the signal transduction pathways involved are unknown. Both high and low doses of ROS up-regulate cellular glutathione and activate DNA-binding to the ARE element.
ACKNOWLEDGEMENTS
This work was supported by the National Heart, Lung, and Blood Institute Grants R01HL73929, R01HL67340 and R01HL72844. The opinions expressed in this document are those of the authors and do not reflect the views of the Uniformed Services University of the Health Science, the Department of Defense or the Federal Government.
Abbreviations
- AP-1
activator protein 1
- ARE
antioxidant response element
- BHK-21
baby hamster kidney fibroblasts
- Cu/Zn-SOD
copper/zinc superoxide dismutase
- FBS
fetal bovine serum
- γGCS
gamma-glutamyl cycteine synthetase
- MAPK
mitogen activated protein kinase
- MnSOD
manganese superoxide dismutase
- NAC
N-acetyl-l-cysteine
- NADH
nicotinamide adenine dinucleotide
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa B
- NOX
NAD(P)H oxidase homolog
- PAEC
pulmonary artery endothelial cells
- ROS
reactive oxygen species
- RPMEC
rat pulmonary microvascular endothelial cell
- SMC
smooth muscle cells
- SOD
superoxide dismutase
- TGFβ1
transforming growth factor beta1
REFERENCES
- Anderson M.T., Staal F.J., Gitler C., Herzenberg L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc Natl Acad Sci U S A. 1994;91:11527–31. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R.S., Shi J., Murad E., Whalen A.M., Sun C.Q., Polavarapu R., Parthasarathy S., Petros J.A., Lambeth J.D. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B.M. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–9. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- Bayraktutan U., Blayney L., Shah A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–11. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Post J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Borutaite V., Brown G.C. Nitric oxide induces apoptosis via hydrogen peroxide, but necrosis via energy and thiol depletion. Free Radic Biol Med. 2003;35:1457–68. doi: 10.1016/j.freeradbiomed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Miller F.J., Jr., Li W.G., Ellingson A.N., Mozena J.D., Chatterjee P., Engelhardt J.F., Zwacka R.M., Oberley L.W., Fang X., Spector A.A., Weintraub N.L. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res. 1999;85:524–33. doi: 10.1161/01.res.85.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Burdon R.H., Alliangana D., Gill V. Endogenously generated active oxygen species and cellular glutathione levels in relation to BHK-21 cell proliferation. Free Radic Res. 1994;21:121–33. doi: 10.3109/10715769409056564. [DOI] [PubMed] [Google Scholar]
- Burdon R.H., Alliangana D., Gill V. Hydrogen peroxide and the proliferation of BHK-21 cells. Free Radic Res. 1995;23:471–86. doi: 10.3109/10715769509065268. [DOI] [PubMed] [Google Scholar]
- Burdon R.H., Gill V., Rice-Evans C. Cell proliferation and oxidative stress. Free Radic Res Commun. 1989;7:149–59. doi: 10.3109/10715768909087937. [DOI] [PubMed] [Google Scholar]
- Burdon R.H., Gill V., Rice-Evans C. Oxidative stress and tumour cell proliferation. Free Radic Res Commun. 1990;11:65–76. doi: 10.3109/10715769009109669. [DOI] [PubMed] [Google Scholar]
- Burdon R.H., Rice-Evans C. Free radicals and the regulation of mammalian cell proliferation. Free Radic Res Commun. 1989;6:345–58. doi: 10.3109/10715768909087918. [DOI] [PubMed] [Google Scholar]
- Chamseddine A.H., Miller F.J., Jr. Gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H2284–9. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- Chang S.C., Kao M.C., Fu M.T., Lin C.T. Modulation of NO and cytokines in microglial cells by Cu/Zn-superoxide dismutase. Free Radic Biol Med. 2001;31:1084–9. doi: 10.1016/s0891-5849(01)00691-8. [DOI] [PubMed] [Google Scholar]
- Chung-man Ho J., Zheng S., Comhair S.A., Farver C., Erzurum S.C. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61:8578–85. [PubMed] [Google Scholar]
- Cullen J.J., Weydert C., Hinkhouse M.M., Ritchie J., Domann F.E., Spitz D., Oberley L.W. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63:1297–303. [PubMed] [Google Scholar]
- Cumming R.C., Andon N.L., Haynes P.A., Park M., Fischer W.H., Schubert D. Protein Disulfide Bond Formation in the Cytoplasm during Oxidative Stress. J Biol Chem. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- Das S.K., White A.C., Fanburg B.L. Modulation of transforming growth factor-beta 1 antiproliferative effects on endothelial cells by cysteine, cystine, and N-acetylcysteine. J Clin Invest. 1992;90:1649–56. doi: 10.1172/JCI116036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K.J. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999;48:41–7. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- Day R.M., Suzuki Y.J., Fanburg B.L. Regulation of glutathione by oxidative stress in bovine pulmonary artery endothelial cells. Antioxid Redox Signal. 2003;5:699–704. doi: 10.1089/152308603770379991. [DOI] [PubMed] [Google Scholar]
- Day R.M., Suzuki Y.J., Lum J.M., White A.C., Fanburg B.L. Bleomycin upregulates expression of gamma-glutamylcysteine synthetase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1349–57. doi: 10.1152/ajplung.00338.2001. [DOI] [PubMed] [Google Scholar]
- Deneke S.M., Fanburg B.L. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–73. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Deneke S.M., Steiger V., Fanburg B.L. Effect of hyperoxia on glutathione levels and glutamic acid uptake in endothelial cells. J Appl Physiol. 1987;63:1966–71. doi: 10.1152/jappl.1987.63.5.1966. [DOI] [PubMed] [Google Scholar]
- Fiers W., Beyaert R., Declercq W., Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–30. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–4. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- Frippiat C., Dewelle J., Remacle J., Toussaint O. Signal transduction in H 2O 2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 2002;33:1334–46. doi: 10.1016/s0891-5849(02)01044-4. [DOI] [PubMed] [Google Scholar]
- Gallagher D.L., Betteridge L.J., Patel M.K., Schachter M. Effect of oxidants on vascular smooth muscle proliferation. Biochem Soc Trans. 1993;21:98S. doi: 10.1042/bst021098s. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W.B. Ca2+-and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–62. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K.K., Harrison D.G. Dual role of reactive oxygen species in vascular growth. Circ Res. 1999;85:562–3. doi: 10.1161/01.res.85.6.562. [DOI] [PubMed] [Google Scholar]
- Griffith O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Grigolo B., Lisignoli G., Toneguzzi S., Mazzetti I., Facchini A. Copper/zinc superoxide dismutase expression by different human osteosarcoma cell lines. Anticancer Res. 1998;18:1175–80. [PubMed] [Google Scholar]
- Guy G.R., Cairns J., Ng S., Tan Y.H. Inactivation of a redox-sensitive protein phosphatase during the early eents of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- Halliwell B. Free radicals, proteins and DNA: oxidative damage versus redox regulation. Biochem Soc Trans. 1996;24:1023–7. doi: 10.1042/bst0241023. [DOI] [PubMed] [Google Scholar]
- Hohler B., Holzapfel B., Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochem Cell Biol. 2000;114:29–37. doi: 10.1007/s004180000160. [DOI] [PubMed] [Google Scholar]
- Hoshikawa Y., Nana-Sinkam P., Moore M.D., Sotto-Santiago S., Phang T., Keith R.L., Morris K.G.K., Tuder T.R.M., Voelkel N.F., Geraci M.W. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genom. 2003;12:209–219. doi: 10.1152/physiolgenomics.00081.2001. [DOI] [PubMed] [Google Scholar]
- Hur G.C., Cho S.J., Kim C.H., Kim M.K., Bae S.I., Nam S.Y., Park J.W., Kim W.H., Lee B.L. Manganese superoxide dismutase expression correlates with chemosensitivity in human gastric cancer cell lines. Clin Cancer Res. 2003;9:5768–75. [PubMed] [Google Scholar]
- Janssen A.M., Bosman C.B., Kruidenier L., Griffioen G., Lamers C.B., van Krieken J.H., van de Velde C.J., Verspaget H.W. Superoxide dismutases in the human colorectal cancer sequence. J Cancer Res Clin Oncol. 1999;125:327–35. doi: 10.1007/s004320050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., O'Donnell V.B., Wood J.D., Broughton J.P., Hughes E.J., Jones O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–34. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Joneson T., Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–4. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- Kinnula V.L., Everitt J.I., Whorton A.R., Crapo J.D. Hydrogen peroxide production by alveolar type II cells, alveolar macrophages, and endothelial cells. Am J Physiol. 1991;261:L84–91. doi: 10.1152/ajplung.1991.261.2.L84. [DOI] [PubMed] [Google Scholar]
- Kyaw M., Yoshizumi M., Tsuchiya K., Kirima K., Suzaki Y., Abe S., Hasegawa T., Tamaki T. Antioxidants inhibit endothelin-1 (1–31)-induced proliferation of vascular smooth muscle cells via the inhibition of mitogen-activated protein (MAP) kinase and activator protein-1 (AP-1) Biochem Pharmacol. 2002;64:1521–31. doi: 10.1016/s0006-2952(02)01349-7. [DOI] [PubMed] [Google Scholar]
- Lando D., Peet D.J., Whelan D.A., Gorman J.J., Whitelaw M.L. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Li P.F., Dietz R., von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation. 1997;96:3602–9. doi: 10.1161/01.cir.96.10.3602. [DOI] [PubMed] [Google Scholar]
- Li P.F., Dietz R., von Harsdorf R. Superoxide induces apoptosis in cardiomyocytes, but proliferation and expression of transforming growth factor-beta1 in cardiac fibroblasts. FEBS Lett. 1999;448:206–10. doi: 10.1016/s0014-5793(99)00370-1. [DOI] [PubMed] [Google Scholar]
- Liu S.L., Lin X., Shi D.Y., Cheng J., Wu C.Q., Zhang Y.D. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002;406:173–82. doi: 10.1016/s0003-9861(02)00430-7. [DOI] [PubMed] [Google Scholar]
- Malafa M., Margenthaler J., Webb B., Neitzel L., Christophersen M. MnSOD expression is increased in metastatic gastric cancer. J Surg Res. 2000;88:130–4. doi: 10.1006/jsre.1999.5773. [DOI] [PubMed] [Google Scholar]
- Marcho z., White J.E., Higgins P.J., Tsan M.-F. Tumor necrosis factor enhances endothelial cell susceptibility to oxygen toxicity: Role of glutathione. Am J Respir Cell Mol Biol. 1991;5:556–562. doi: 10.1165/ajrcmb/5.6.556. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986;137:3295–8. [PubMed] [Google Scholar]
- Murphy H.S., Yu C., Quddus J. Functional expression of NAD(P)H oxidase p47 in lung microvascular endothelial cells. Biochem Biophys Res Commun. 2000;278:584–9. doi: 10.1006/bbrc.2000.3848. [DOI] [PubMed] [Google Scholar]
- Murrell G.A., Francis M.J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–65. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Schey K.L., Takagahara S., Kontani K., Katada T., Urano Y., Nagano T., Nagao T., Kurose H. Activation mechanism of Gi and Go by reactive oxygen species. J Biol Chem. 2002;277:9036–42. doi: 10.1074/jbc.M107392200. [DOI] [PubMed] [Google Scholar]
- Palazzotti B., Pani G., Colavitti R., De Leo M.E., Bedogni B., Borrello S., Galeotti T. Increased growth capacity of cervical-carcinoma cells over-expressing manganous superoxide dismutase. Int J Cancer. 1999;82:145–50. doi: 10.1002/(sici)1097-0215(19990702)82:1<145::aid-ijc24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Parinandi N.L., Kleinberg M.A., Usatyuk P.V., Cummings R.J., Pennathur A., Cardounel A.J., Zweier J.L., Garcia J.G., Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- Perner A., Andresen L., Pedersen G., Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–6. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Plymate S.R., Haugk K.H., Sprenger C.C., Nelson P.S., Tennant M.K., Zhang Y., Oberley L.W., Zhong W., Drivdahl R., Oberley T.D. Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene. 2003;22:1024–34. doi: 10.1038/sj.onc.1206210. [DOI] [PubMed] [Google Scholar]
- Preston T.J., Muller W.J., Singh G. Scavenging of extracellular H 2O 2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J Biol Chem. 2001;276:9558–64. doi: 10.1074/jbc.M004617200. [DOI] [PubMed] [Google Scholar]
- Pustovidko A.V., Potselueva M.M., Evtodienko Y.V. Generation of reactive oxygen species by polymorphonuclear leukocytes and its modulation by calcium ions during tumor growth. IUBMB Life. 2000;50:69–73. doi: 10.1080/15216540050176629. [DOI] [PubMed] [Google Scholar]
- Remacle J., Lambert D., Raes M., Pigeolet E., Michiels C., Toussaint O. Importance of various antioxidant enzymes for cell stability. Confrontation between theoretical and experimental data. Biochem J. 1992;286:41–6. doi: 10.1042/bj2860041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle J., Raes M., Toussaint O., Renard P., Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316:103–22. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Schimmel M., Bauer G. Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene. 2002;21:5886–96. doi: 10.1038/sj.onc.1205740. [DOI] [PubMed] [Google Scholar]
- Sorescu D., Griendling K.K. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- Staal F.J., Anderson M.T., Staal G.E., Herzenberg L.A., Gitler C. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc Natl Acad Sci U S A. 1994;91:3619–22. doi: 10.1073/pnas.91.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y.A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Sundaresan M., Yu Z.X., Ferrans V.J., Sulciner D.J., Gutkind J.S., Irani K., Goldschmidt-Clermont P.J., Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318(Pt 2):379–82. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh A., Guedez L., Moreb J., Zucali J. Overexpression of manganese superoxide dismutase promotes survival in cell lines after doxorubicin treatment. Br J Haematol. 2003;120:457–63. doi: 10.1046/j.1365-2141.2003.04074.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.J., Cleemann L., Abernethy D.R., Morad M. Glutathione is a cofactor for H2O2-mediated stimulation of Ca2+-induced Ca2+ release in cardiac myocytes. Free Radic Biol Med. 1998;24:318–25. doi: 10.1016/s0891-5849(97)00227-x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.J., Forman H.J., Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–85. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Takada Y., Hachiya M., Park S.H., Osawa Y., Ozawa T., Akashi M. Role of reactive oxygen species in cells overexpressing manganese superoxide dismutase: mechanism for induction of radioresistance. Mol Cancer Res. 2002;1:137–46. [PubMed] [Google Scholar]
- Thannickal V.J., Day R.M., Klinz S.G., Bastien M.C., Larios J.M., Fanburg B.L. Rasdependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. Faseb J. 2000;14:1741–8. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Thannickal V.J., Hassoun P.M., White A.C., Fanburg B.L. Enhanced rate of H 2O 2 release from bovine pulmonary artery endothelial cells induced by TGF-beta 1. Am J Physiol. 1993;265:L622–6. doi: 10.1152/ajplung.1993.265.6.L622. [DOI] [PubMed] [Google Scholar]
- Veal E.A., Findlay V.J., Day A.M., Bozonet S.M., Evans J.M., Quinn J., Morgan B.A. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–39. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Weening R.S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975;85:245–52. [PubMed] [Google Scholar]
- White A.C., Maloney E.K., Lee S.L., Lanzillo J.J., Fanburg B.L. Reduction of endothelial cell related TGFbeta activity by thiols. Endothelium. 1999;6:231–9. doi: 10.3109/10623329909053413. [DOI] [PubMed] [Google Scholar]
- Wild A.C., Mulcahy R.T. Regulation of γ-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Review Free Rad Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schroder E., Robin H.J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Xu D., Rovira II, Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell. 2002;2:251–2. doi: 10.1016/s1534-5807(02)00132-6. [DOI] [PubMed] [Google Scholar]
- Yang J.Q., Li S., Huang Y., Zhang H.J., Domann F.E., Buettner G.R., Oberley L.W. V-Ha-Ras overexpression induces superoxide production and alters levels of primary antioxidant enzymes. Antioxid Redox Signal. 2001;3:697–709. doi: 10.1089/15230860152543032. [DOI] [PubMed] [Google Scholar]
- Yang S., Madyastha P., Bingel S., Ries W., Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–8. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- Zafari A.M., Ushio-Fukai M., Akers M., Yin Q., Shah A., Harrison D.G., Taylor W.R., Griendling K.K. Role of NADH/NADPH oxidase-derived H 2O 2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–95. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zhao R., Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem. 2002;277:39456–62. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- Zulueta J.J., Yu F.-S., Hertig I.A., Thannickal V.J., Hassoun P.M. Release of hydrogen peroxide in response to hypoxia-reoxygenation: role of an NAD(P)H oxidase-like enzyme in endothelial cell plasma membrane. Am J Respir Cell Mol Biol. 1995;12:41–49. doi: 10.1165/ajrcmb.12.1.7529030. [DOI] [PubMed] [Google Scholar]