Abstract
Persistent organic pollutants (POPs) are long-lived toxic organic compounds and are of major concern for human and ecosystem health. Although the use of most POPs is banned in most countries, some organochlorine pesticides are still being used in several parts of the world. Although environmental levels of some POPs such as polychlorinated biphenyls (PCBs) have declined, newly emerging POPs such as polybrominated diphenyl ethers (PBDEs) have been increasing considerably. Exposure to POPs has been associated with a wide spectrum of effects including reproductive, developmental, immunologic, carcinogenic, and neurotoxic effects. It is of particular concern that neurotoxic effects of some POPs have been observed in humans at low environmental concentrations. This review focuses on PCBs as a representative chemical class of POPs and discusses the possible mode(s) of action for the neurotoxic effects with emphasis on comparing dose-response and structure-activity relationships (SAR) with other structurally related chemicals. There is sufficient epidemiological and experimental evidence showing that PCB exposure is associated with motor and cognitive deficits in humans and animal models. Although several potential mode(s) of actions were postulated for PCB-induced neurotoxic effects, changes in neurotransmitter systems, altered intracellular signalling processes, and thyroid hormone imbalance are predominant ones. These three potential mechanisms are discussed in detail in vitro and in vivo. In addition, SAR was conducted on other structurally similar chemicals to see if they have a common mode(s) of action. Relative potency factors for several of these POPs were calculated based on their effects on intracellular signalling processes. This is a comprehensive review comparing molecular effects at the cellular level to the neurotoxic effects seen in the whole animal for environmentally relevant POPs.
Keywords: Neurotoxicity, persistent organic pollutants, mode of action, intracellular signaling, polychlorinated biphenyls, neurotransmitters, thyroid hormones, polybrominated diphenyl ethers, structure-activity relationships
INTRODUCTION
Tens of thousands of chemicals are produced or handled in a modern industrial society. Some of these chemicals are generated unintentionally as contaminants in the course of various manufacturing processes. Both laboratory and epidemiological studies have shown that many of these chemicals are hazardous to human health if present in excessive quantities in our food or the environment in which we live. Persistent organic pollutants (POPs) are long-lived toxic organic compounds such as polychlorinated biphenyls (PCBs), organochlorine pesticides, and dioxins. These chemicals are major concern for human and ecosystem health due to their high degree of persistence. Although the use of most POPs are banned in most countries, some organochlorine pesticides are still being used in parts of the world, which could lead to the environmental pollution of the neighboring regions, or even the entire world. In addition, several tons of these POPs are still available for release into the environment (Lang, 1992, ATSDR, 1999). For example, it has been estimated that more than 1.2 × 109 kg of PCBs have been manufactured throughout the world; 3.7 × 108 kg of PCBs have already been released into the environment, and an additional 7.8 × 108 kg are still available or deposited in different ways (Lang, 1992). These POPs could be released with time, remain in the environment for long period of time due to their high persistence, and bioaccumulate in the food web. Even though concentrations of POPs in the natural environment have fallen, PCBs and other long-known POPs cannot be dismissed as being of no significance to human health (SEPA, 1998). Since PCBs represent a significant group of POPs, the focus of this review will be on this group of chemicals discussing the neurotoxic effects and possible mode(s) of action with emphasis on dose-response in vitro, in vivo, and structure-activity relationships.
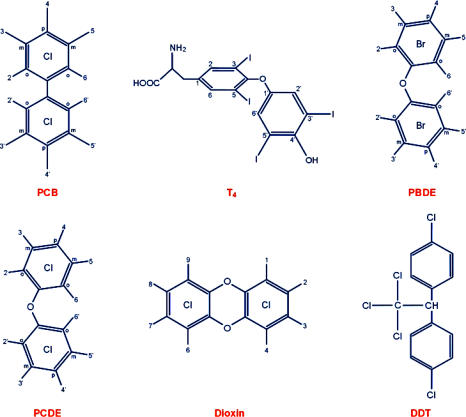
Polychlorinated biphenyls (PCBs) belong to a group of pollutants called polyhalogenated aromatic hydrocarbon family. This family includes other pollutants such as polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans (PCDF), polychlorinated diphenyl ethers, DDT, and polybrominated diphenyl ethers (PBDEs) (Figure 1). PCBs exist as mixtures containing up to 209 possible congeners and are ubiquitous environmental contaminants resulting from intensive industrial use and inadequate disposal over past decades (Erickson, 1986). PCB mixtures as well as congeners possess a surprising array of biological activity leading to toxicity (WHO, 1993). It is known that some PCBs and other halogenated hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) produce some of their biological effects through a receptor-mediated response by binding to the cytosolic aryl hydrocarbon (Ah) receptor followed by induction of a number of genes (Safe, 1994; Okey et al., 1994). It has been proposed that non-ortho substitutions on the biphenyl ring (lateral substitutions at the meta- and para- positions) promote coplanarity and this is associated with the TCDD-like toxic effects of some coplanar PCB congeners (McKinney and Waller, 1994). Ah receptor involvement is associated with reproductive, immunologic, teratogenic, and carcinogenic effects of PCBs (Safe, 1994; Kafafi et al., 1993). The neurotoxic effects of PCBs, however, might not be entirely mediated through the Ah receptor (Schantz, 1996; Seegal, 1996; Tilson and Kodavanti, 1997, 1998; Kodavanti, 2004).
FIGURE 1.
Structural similarities between polyhalogenated aromatic hydrocarbons and thyroxine (T4). The numbers on the structures indicate the possible position for halogens. o = ortho; m = meta; p = para; PCB = polychlorinated biphenyl; T4 = Thyroxine; PBDE = polybrominated diphenyl ether; PCDE = polychlorinated dipheyl ether; DDT = 1,1,1-trichloro-2,2-bis[p-chlorophenyl]-ethane.
HUMAN (EPIDEMIOLOGICAL STUDIES) EVIDENCE OF NEUROTOXICITY BY POPs EXPOSURE
There is sufficient evidence that PCBs can cause neurotoxicity in humans (see Table 1) and it is believed that in utero exposure is more important than lactational exposure in causing the neurotoxic effects. The toxicological properties of PCBs became evident after several well documented episodes of accidental exposures (Tilson et al., 1990; Rogan and Gladen, 1992; Sinks et al., 1992). There have been two major accidental PCB poisoning incidents; “Yusho” occurred in 1968 in Japan and “YuCheng” occurred ten years later in Taiwan. These incidents resulted in several health problems including chloracne, numbness, weakness in limbs, decreased peripheral nerve conduction velocities, developmental delays, and speech problems (Kuratsune et al., 1971; 1972; Rogan et al., 1988). The offspring of women who had ingested PCB-contaminated ricebran cooking oil in Taiwan and Japan were perinatally exposed to PCBs. The Japanese children were hypoactive (Harada, 1976) while the Taiwanese children were hyperactive and had more behavior problems compared with unexposed controls (Chen et al., 1994). When the children were tested at ages between 7 and 12, the exposed Taiwanese children also had significantly lower verbal and full-scale IQs. They also showed prolongation of, and a significant reduction in the amplitude of auditory evoked potentials (Chen and Hsu, 1994). The YuCheng and Yusho incidents were also attributed to PCDF exposure, since rice oils were cross-contaminated with PCDFs and PCBs.
TABLE 1.
Evidence of neurotoxicity in humans and animals (monkeys, rats, and mice) following exposure to PCBs.
| NEUROTOXICITY IN HUMANS | ||
|---|---|---|
| PCB mixtures/congeners (exposure) | Effect | References |
| Mixture of PCBs and PCDFs (adult and developmental) | Decreased peripheral nerve conduction velocities, developmental delays, speech problems | Kuratsune et al., 1971, 1972; |
| Hypoactive | Rogan et al., 1988 | |
| Hyperactive | Harada, 1976 | |
| Chen et al., 1994 | ||
| Mixture of PCBs (developmental) | Learning and memory deficits | Jacobson et al., 1990; |
| Poor performance in psychomotor activities | Jacobson & Jacobson, 1996 | |
| Rogan and Gladen, 1992; | ||
| Winneke et al., 1998 | ||
| NEUROTOXICITY IN MONKEYS | ||
| PCB mixtures/congeners (exposure) | Effect | References |
| PCB mixture (developmental) | Locomotor hyperactivity | Bowman et al., 1981 |
| Long-term changes in cognitive function | Schantz et al., 1989 | |
| NEUROTOXICITY IN RATS | ||
| PCB mixtures/congeners (exposure) | Effect | References |
| PCB mixture (adult) | Decreased motor activity | Nishida et al., 1997 |
| PCB mixture (developmental) | Transient changes in functional observation battery | Bushnell et al., 2002 |
| Impaired radial arm maze | Roegge et al., 2000 | |
| Affected long term potential | Gilbert et al., 2000 | |
| Low frequency hearing deficits | Crofton et al., 2000; | |
| Herr et al., 2001 | ||
| Ortho congeners (developmental) | Hyperactive and impulsive | Holene et al., 1998 |
| NEUROTOXICITY IN MICE | ||
| PCB mixtures/congeners (exposure) | Effect | References |
| PCB mixture (developmental) | Increased motor activity | Storm et al., 1981 |
| Impaired learning | ||
| Non-ortho congener (developmental) | Hyperactivity and reduced visual discrimination learning | Agrawal et al., 1981 |
| Ortho congener (developmental) | Persistent aberrations in spontaneous behavior | Eriksson and Fredriksson, 1996 |
In addition to these accidental poisoning incidents, there were several epidemiological studies. Recently, Longnecker et al. (2003) compared PCB levels across ten epidemiological studies on human neurodevelopment. Jacobson et al. (1990) and Jacobson and Jacobson (1996) reported learning and memory deficits in children born to mothers, who had ingested fish, thought to be contaminated with PCBs. Rogan and Gladen (1992) reported that prenatal PCB exposure was associated with poorer performance on the Psychomotor Index from Bayley Scales of Infant Development in children from general population in North Carolina. Studies from The Netherlands (Patandin et al., 1999) also indicated that in utero exposure to “background” PCB concentrations is associated with poorer cognitive functioning in preschool children. Studies from Germany (Winneke et al., 1998; Walkowiak et al., 2001) also indicated that PCB exposure is associated with deficits in mental and motor scores in children up to 7-months of age. Studies from Faroese birth cohort in Denmark indicated that cord PCB concentration was associated with deficits in learning, however, these results may be confounded by mercury and suggest a possible interaction between these two neurotoxicants (Grandjean et al., 2001). These accidental poisoning incidents and epidemiological studies suggest that the nervous system, especially during development, is sensitive to exposure to PCBs and related chemicals.
EVIDENCE OF NEUROTOXICITY IN ANIMALS BY POPs EXPOSURE
Several studies using experimental animals support the results observed in humans (for reviews, see Seegal and Schantz, 1994; Tilson and Kodavanti, 1997; Kodavanti, 2004; and Table 1). Behavioral changes and learning deficits have been observed in monkeys (Schantz et al., 1989), rats (Pantaleoni et al., 1988), and mice (Eriksson and Fredriksson, 1996). Neurobehavioral changes were observed in adult animals following acute exposure (Nishida et al., 1997) as well as in animals exposed perinatally during development (Schantz et al., 1995). In general, neurotoxicity studies have used different PCB dosing (e.g., mixtures versus individual congener), different dosing regimens (gestational, lactational, perinatal or adults), and different dosing pattern (acute versus chronic or repeated), and these methodological factors are important in explaining some inconsistencies in the literature. In many of these animal studies, functional deficits were observed in the absence of reduced body weight or overt signs of toxicity. Animals that were prenatally, postnatally or perinatally exposed to PCBs showed many of the behavioral characteristics indicative of Attention Deficit Hyperactivity Disorder (AD/HD). For example, in utero exposure of mice led to increased motor activity and impaired learning (Storm et al., 1981), combined pre- and post-natal exposure lead to higher activity levels and reduced visual discrimination learning in rats (Agrawal et al., 1981). Holene et al. (1998) showed that male rats exposed to 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) during lactation became hyperactive and impulsive. Rhesus monkeys exposed throughout gestation and lactation showed locomotor hyperactivity during their first year of life (Bowman et al., 1981). Mice exposed during brain “growth spurt” on postnatal day 10 to 2,4,4′-trichlorobiphenyl and 2,2′,5,5′-tetrachlorobiphenyl exhibited persistent aberrations in spontaneous behavior while 2,3′,4,4′,5-penta – and 2,3,3′,4,4′,5-hexa-chlorobiphenyls did not (Eriksson and Fredriksson, 1996). Studies by Bushnell et al (2002) indicated that perinatal exposure to Aroclor 1254 caused only transient changes in some measures of functional observational battery without any changes in motor activity.
Apart from effects on motor activity, PCBs have been shown to decrease cognitive function in rats, non-human primates, and mice. Holene et al. (1995) reported that developmental exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) and 2,3′,4,4′,5-pentachlorobiphenyl (PCB 118) at doses that did not produce maternal or fetotoxicity interfered with visual discrimination learning of rats in an operant task. Schantz et al (1995) reported that developmental exposure to ortho-substituted PCBs significantly interfered with acquisition of the spatial alternation task in females, but not in males. Studies also indicated that gestational and lactational exposure to a commercial PCB mixture, Aroclor 1254, impaired radial arm maze performance in male rats (Roegge et al., 2000) and affected long-term potentiation in the hippocampus (Niemi et al., 1998; Gilbert et al., 2000). In non-human primates, developmental exposure to commercial PCB mixtures (Aroclor 1016 or Aroclor 1248) resulted in long-term changes in cognitive function (Schantz et al., 1989). Mice exposed to 2,2′,5,5′-tetrachlorobiphenyl on postnatal day 10 (during brain “growth spurt”) exhibited deficits in learning and memory while 2,3′,4,4′,5-penta- and 2,3,3′,4,4′,5-hexa-chlorobiphenyls did not (Eriksson and Fredriksson, 1996).
Other effects seen with developmental exposure to PCBs include sensory deficits. The most significant effect seen with developmental exposure to PCBs was deficits in hearing; the auditory threshold at low frequency (1 kHz) was significantly increased (Crofton et al., 2000; Herr et al., 2001). These effects of PCBs on hearing deficits seen at a very low frequency threshold in rats may not be of biological significance in humans. Herr et al (2001) also indicated that developmental PCB exposure has no significant effect on visual-, somatosensory- or peripheral nerve-evoked potentials. Geller et al. (2001) reported gender-specific effects of PCBs on neurobehavioral development on measures of acquisition and sensory function, as seen with some behavioral endpoints. These animal studies clearly indicated that developmental exposure to PCBs resulted in cognitive dysfunction, motor deficits, and hearing loss.
POSSIBLE MODE(S) OF ACTION
Although there is both epidemiological and experimental evidence that developmental exposure to PCBs causes motor and cognitive deficits in humans and animals (see Table 1), the underlying cellular or molecular mechanisms are not known (Kodavanti and Tilson, 1997, 2000; Tilson and Kodavanti, 1997). There are several potential mechanisms postulated for the developmental neurotoxicity of PCBs. This review will focus on three predominant hypotheses; changes in neurotransmitters, altered intracellular signaling processes, and thyroid hormone imbalance.
Dose-response effects of POPs on Neurotransmitters
It has been hypothesized that changes in neurotransmitters such as dopamine (DA) levels may be associated with the observed neurobehavioral changes. A study by Chou et al. (1979) in weaning mice indicated that approximately half of the surviving offspring exhibited a syndrome of rapid spinning and head bobbing when the moms were exposed to 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) from gestational days 10–16, at a dose of 32 mg/kg/day. A similar observation was made by Agrawal et al (1981) indicating that mice exposed developmentally to PCB 77 had elevated levels of motor activity and these changes were associated with decreased DA concentrations and receptor binding sites in the corpus striatum. In contrast, studies by Seegal (1994) found that developmental exposure to commercial PCB mixture, Aroclor 1016, significantly increased DA concentrations in substantia nigra, caudate nucleus and nucleus accumbens, while there were no effects on DA metabolites, nor-epinephrine, serotonin or its metabolites. These observations were different from those seen after acute and repeated exposure of Aroclor mixtures in adults where significant decreases in DA concentrations were observed (Seegal et al., 1986, Seegal et al., 1991). However, in utero and lactational exposure to 2,2′,4,4′-tetrachlorobiphenyl (ortho substituted congener, PCB 47) resulted in a decrease in DA concentrations in frontal cortex and caudate nucleus that persisted into adulthood while exposure to PCB 77 (non-ortho substituted congener) resulted in increases in DA concentrations in the same brain regions (Seegal et al., 1997). The effects of PCBs were not restricted to dopamine function only. Morse et al. (1996a) reported that developmental exposure to a PCB mixture, Aroclor 1254, resulted in persistent serotonergic effects, while no effects on dopaminergic system were observed. Also, Juarez de Ku et al. (1994) reported that developmental exposure to a PCB mixture affected hippocampal and forebrain cholinergic system. Eriksson and Fredriksson (1996) reported decreases in cholinergic nicotinic receptors in mice with deficits in spontaneous motor activity and learning following exposure to PCBs during brain “growth spurt”.
These in vivo observations on neurotransmitters were accompanied by several in vitro studies to understand the mechanism and structure-activity relationships (SAR) of PCB congeners. Seegal and his colleagues focused on dopamine. As seen in vivo with ortho-substituted congeners and Aroclor mixtures, DA concentrations in PC12 cells also decreased in a concentration-dependent manner following exposure in vitro. Studies with 43 PCB congeners to understand SAR found that congeners with ortho- or ortho- and para-chlorine substitutions were most potent in decreasing DA concentrations in PC12 cells (Shain et al., 1991; Table 2). Further studies investigated the mechanism by which PCB congeners inhibited DA synthesis using neuroblastoma cells (N1E-N115), which lack the enzyme, aromatic amino acid decarboxylase. This decarboxylase enzyme converts 3,4-dihydroxylphenylalanine (DOPA), formed by hydroxylation of tyrosine, to dopamine. These studies indicated that PCBs appear to decrease cellular DA concentrations by inhibiting the activity of the rate-limiting enzyme, tyrosine hydroxylase (Seegal et al., 1991). Other studies with rat striatal slices indicated that ortho-substituted PCB congeners inhibited tyrosine hydroxylase activity in vitro (Choksi et al., 1997). However, these in vitro results were not confirmed by in vivo studies in adult rats where tyrosine hydroxylase immunoreactivity in forebrain or enzyme activity in striatum was not significantly altered following repeated exposure to Aroclor 1254 (Kodavanti et al., 1998a). In the same animals, however, neurotransmitter levels in striatum were slightly altered by PCB exposure. Recent studies also indicated that tyrosine hydroxylase expression was not affected by acute exposure to Aroclors 1016 or 1260 (Richardson and Miller, 2004). There are other studies indicating effects of PCBs on the high affinity uptake of the neurotransmitters in to rat brain synaptic vesicles and synaptosomes (Mariussen et al., 1999; Mariussen and Fonnum, 2001). These studies indicated that several ortho-substituted PCBs inhibited neurotransmitter uptake in to rat brain synaptosomes; dopamine uptake was inhibited more efficient that that of glutamate. On the other hand, non-ortho PCBs did not have any effect supporting the SAR of Shain et al. (1991). In addition, recent reports indicated that inhibition of vesicular monoamine transporter might be involved in the reductions of synaptosomal dopamine content by PCBs (Bemis and Seegal, 2004; Richardson and Miller, 2004). Based on these in vivo and in vitro studies, a possible role of neurotransmitters in PCB neurotoxicity could not be ruled out.
TABLE 2.
The reason for selecting different polychlorinated biphenyl (PCB) mixtures, polybrominated diphenyl ether (PBDE) mixures, congeners, dioxin, and furan to study the structure-activity relationships (SAR)†.
| PCB MIXTURES | ||
|---|---|---|
| Test chemical | IUPAC # | Reason for selecting |
| Aroclor 1016 | Effective, lightly chlorinated, ortho-congeners detected in brain. | |
| Aroclor 1254 | Heavily chlorinated, ortho-congeners detected in brain, well-studied. | |
| Aroclor 1260 | Heavily chlorinated, ortho-congeners detected in brain, well-studied, Closest one for environmental mixture. | |
| PCB CONGENERS∗ | ||
| Test chemical | IUPAC # | Reason for selecting |
| 2,2′ | 4 | Neuroactive in vitro, Found in soil/water samples |
| 3,3′ | 11 | Non-ortho, but neuroactive in vitro. |
| 3,5 | 14 | Neuroactive in vitro. |
| 4,4′ | 15 | Non-neuroactive in vitro. |
| 2,2′,6 | 19 | Congener with three ortho's, but no lateral substitutions. |
| 2,4,4′ | 28 | Neuroactive in vitro, major congener detected in brain and human milk; detected in sewage sludge, drinking water and human fat. |
| 2,2′,4,4′ | 47 | Neuroactive in vitro. |
| 2,2′,4,6 | 50 | Neuroactive in vitro. |
| 2,2′,4,6′ | 51 | Neuroactive in vitro. |
| 2,2′,5,5′ | 52 | Detected in sewage sludge, drinking water, fish, grass, cow milk, and human fat. |
| 2,2′,6,6′ | 54 | Fully saturated ortho-congener that is non-neuroactive |
| 3,3′,4,4′ | 77 | Non-neuroactive in vitro, but neuroactive in vivo. Detected in human adipose tissue & breast milk at low quantity; Found in Lake Michigan fish |
| 3,3′,5,5′ | 80 | Fully saturated meta-congener |
| 2,2′,4,6,6′ | 104 | Neuroactive in vitro. |
| 2,3,3′,4,4′ | 105 | Detected in Lake Michigan fish, Found in most dairy products. |
| Major mono-ortho congener detected in rat brain. | ||
| 2,3′,4,4′,5 | 118 | Found in serum and adipose tissue of transformer repair workers. |
| Found in all dairy products. Major mono-ortho congener detected in rat brain. | ||
| 3,3′,4,4′,5 | 126 | Non-neuroactive in vitro. Detected in human adipose tissue and breast milk at low quantity. |
| 2,2′,3,3′,4,4′ | 128 | Detected in human fat, cow milk, eels and sewage sludge. |
| 2,2′,3,3′,5,5′ | 133 | Interest with respect to SAR. |
| 2,2′,3,3′,6,6′ | 136 | Interest with respect to SAR. |
| 2,2′,4,4′,5,5′ | 153 | Detected in rodent brain in vivo, Detected in human adipose tissue and breast milk; Found in Lake Ontario fish, meat products & drinking water. |
| 2,3,3′,4,4′,5 | 156 | Major mono-ortho congener detected in rat brain. |
| 3,3′,4,4′,5,5′ | 169 | Detected in human adipose tissue and breast milk at low quantity. |
| 2,2′,3,4,4′,5,5′ | 180 | Detected in human adipose tissue, serum and breast milk. Found in all meat and fish products. Found in drinking water. |
| POLYCHLORINATED DIBENZOFURANS | ||
| Test chemical | IUPAC # | Reason for selecting |
| 1,2,3,7,8 | Commonly found contaminant in breast milk, soil samples, fish etc. | |
| POLYCHLORINATED DIBENZODIOXINS | ||
| Test chemical | IUPAC # | Reason for selecting |
| 2,3,7,8 | Commonly found environmental contaminant. Most active one in dioxins. | |
| POLYCHLORINATED DIPHENYL ETHER MIXTURES, CONGENERS, AND DDT | ||
| Test chemical | IUPAC # | Reason for selecting |
| Nitrofen | a widely used herbicide | |
| o,p′-DDT | a widely used insecticide | |
| p,p′-DDT | a widely used insecticide | |
| Diphenyl ether | selected for understanding SAR with PCBs | |
| 4,4′ | selected for understanding SAR with PCBs | |
| 2,4,4′ | selected for understanding SAR with PCBs | |
| 3,3′,4,4′ | selected for understanding SAR with PCBs | |
| 2,2′4,4′,5 | selected for understanding SAR with PCBs | |
| 2,3′,4,4′,5 | selected for understanding SAR with PCBs | |
| POLYBROMINATED DIPHENYL ETHER MIXTURES AND CONGENERS | ||
| Test chemical | IUPAC # | Reason for selecting |
| DE-71 | Widely used penta mixture in foam materials | |
| DE-79 | Widely used octa mixture in textiles and insulating materials | |
| 2.2′,4,4′ | 47 | Widely detected congener in biological and environmental samples |
| 2,2′,4,4′,5 | 99 | Widely detected congener in biological and environmental samples |
Note:
The numbers under congeners category indicate the position of chlorines on the biphenyl ring.
Source: See Erickson (1986), Safe et al. (1987), Barnes (1991), and WHO (1993). (Adapted from Kodavanti and Tilson, Neurotoxicology 18: 425–442, 1997).
Dose-response effects of POPs on intracellular signaling
Intracellular signaling or signal transduction can be defined as a mechanism by which extracellular signals are transferred to the cytosol and nucleus of the cell. These signals are essential not only for the function of the nervous system, but also play a key role in nervous system development (Murphy et al., 1987; Girard and Kuo, 1990). Any interference with these processes would have the potential for profound effects on the function of neuron as well as their development. Growth factors, neurotransmitters, and hormones serve as first messengers to transfer information from one cell to another by interacting with specific cell membrane receptors. This interaction leads to activation or inhibition of specific enzymes and/or opening of ion channels, which results in changes in intracellular metabolism, leading to a variety of effects including activation of protein kinases and transcription factors. These intracellular pathways can be activated by separate receptors, and there is significant cross talk between the receptor signals, so they can control and modulate each other. In recent years, investigators have considered a number of signal transduction pathways as potential targets for neurotoxicants (Costa, 1998; Nihei et al., 2001; Limke et al., 2003). Neurotoxicants may alter any step beginning from the extracellular stimulus all the way to the transcriptional level leading to alterations in the structure or function of the nervous system. Disrupted intracellular signaling is a potential mechanism by which chemicals might alter nervous system function either directly or through alterations in nervous system morphology. Thus, brain function and/or morphology may be affected by neurotoxicants through changes in intracellular signaling at critical phases during development (Kodavanti, 2004).
It has been hypothesized that PCBs exert their neurotoxic effects by disrupting intracellular signaling/second messenger homeostasis. This hypothesis was based on the following reasons: a) the most significant neurotoxic effects of PCBs seen in humans are learning and memory deficits (Jacobson and Jacobson, 1996; Patandin et al., 1999); b) laboratory studies indicate that PCBs inhibit long-term potentiation (LTP, a form of synaptic plasticity) and impair learning/memory in vivo (Schantz et al., 1995; Niemi et al., 1998; Gilbert et al., 2000); c) LTP is often described as a physiological model for neuronal development, learning and memory (Lynch, 1998); d) second messengers such as calcium, inositol phosphates, PKC, arachidonic acid (AA), and nitric oxide synthase (NOS) have been shown to modulate LTP and play key roles in neuronal development (Lynch, 1998); and e) epidemiological studies indicate that infants born to mothers who consumed contaminated cooking oil in Yusho, Japan showed abnormal calcification of the skull indicating that PCBs/PCDFs might interfere with calcium metabolism (Yamashita and Hayashi, 1985).
A number of studies have been conducted both in vitro using neuronal cultures as well as brain homogenate preparations and in vivo which involved developmental exposure to a PCB mixture, Aroclor 1254, to understand the role of second messengers in the PCB-induced neurotoxicity. In vitro studies have been conducted with prototypic ortho (2,2′-dichlorobiphenyl, DCB) and non-ortho (3,3′,4,4′,5-pentachlorobiphenyl; PeCB or 4,4′-DCB) PCBs followed by an extensive analysis of structure-activity relationships with more than 35 PCB congeners and several analogs (Tables 2–4). PCB-induced alterations in intracellular free Ca2+([Ca2+]i) measured with a fluorescent dye (Fluo-3AM) were reported for the first time; the ortho-substituted 2,2′-DCB was more effective than the non-ortho-substituted 3,3′,4,4′,5-PeCB (Kodavanti et al., 1993). The increase in [Ca2+]i was slow, and a steady rise was observed with time (Kodavanti et al., 1993). Follow up studies confirmed these observations in cerebellar granule neurons (Carpenter et al., 1997; Mundy et al., 1999; Bemis and Seegal, 2000), cortical neurons (Inglefield and Shafer, 2000; Inglefield et al., 2002), and in human granulocytes (Voie and Fonnum, 1998). Studies characterizing the mechanisms by which PCBs increase [Ca2+]i indicated that 2,2′-DCB was an inhibitor of 45Ca2+-uptake by mitochondria and microsomes with IC50 (concentration which inhibits control activity by 50%) values of 6–8 μM. 3,3′,4,4′,5-PeCB inhibited Ca2+-sequestration, but the effects were much less than those produced by equivalent concentrations of 2,2′-DCB. Further studies indicated that Aroclor 1254 inhibited both microsomal and mitochondrial Ca2+-sequestration uniformly in selected brain regions, however, inhibition of microsomal Ca2+-sequestration increased with age (Sharma et al., 2000). Synaptosomal Ca2+-ATPase, involved in Ca2+-extrusion process, was inhibited by 2,2′-DCB, but not by 3,3′,4,4′,5-PeCB (Kodavanti et al., 1993). Further structure-activity relationship (SAR) studies indicated that congeners that are noncoplanar inhibited 45Ca2+-uptake by microsomes and mitochondria while coplanar congeners did not (Kodavanti et al., 1996a).
TABLE 4.
Relative potency of POPs on protein kinase C (PKC) translocation, determined by measuring increases in [3H]PDBu binding in rat cerebellar granule cells in vitro. The congeners vary with different position and number of halogens.
| POLYCHLORINATED BIPHENYL MIXTURES | |||
|---|---|---|---|
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| Aroclor 1016 | Aroclor 1260 | ||
| Aroclor 1254 | |||
| (Lot # 6024) | |||
| Aroclor 1254 | |||
| (Lot # 124–191) | |||
| PCB CONGENERS | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| 2,2′ | 3,3′ | 2,4,4′ | 4,4′ |
| 2,3,4 | 3,5 | 2,4,4′,5 | 2,2′,6,6′ |
| 2,2′,4,6 | 2,2′,6 | 2,2′,4,4′,5 | 3,3′,4,4′ |
| 2,2′,4,6′ | 2,2′,4,4′ | 2,2′,4,4′,6 | 3,3′,4,4′,5 |
| 2,2′,5,5′ | 3,3′,5,5′ | 2,3′,4,4′,5 | 3,3′,4,5,5′ |
| 2,2′,5,6′ | 2,3,3′,4,4′ | 2,2′,3,3′,4,4′ | 3,3′,4,4′,5,5′ |
| 2,2′,3,3′,4 | 2,2′,3,3′,6,6′ | 2,2′,3,3′,5,5′ | 2,2′,3,4,4′,5,5′ |
| 2,2′,3,4,4′ | 2,2′4,4′,5,5′ | ||
| 2,2′,3,5′,6 | 2,3,3′,4,4′,5 | ||
| 2,2′,4,5,5′ | 2,2′,3,3′,5,6,6′ | ||
| 2,2′,4,6,6′ | |||
| 2,3,3′,4′,6 | |||
| HYDROXY-PCBS | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| 2′,4′,6′ | 2′,5′ | 3,5 | |
| 2,2′,5′ | 3,4′,5 | ||
| 2,2′,4′,5,5′ | |||
| POLYCHLORINATED DIBENZOFURAN | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| 1,2,3,7,8 | |||
| POLYCHLORINATED DIBENZODIOXIN | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| 2,3,7,8∗ | |||
| POLYCHLORINATED DIPHENYL ETHER MIXTURES, CONGENERS, AND DDT | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| Nitrofen | |||
| o,p′-DDT | |||
| p,p′-DDT | |||
| 2,4,4′ | 4,4′ | 3,3′,4,4′ | |
| Diphenyl ether | 2,2′4,4′,5 | ||
| 2,3′,4,4′,5 | |||
| POLYBROMINATED DIPHENYL ETHER MIXTURES AND CONGENERS | |||
| Very active (E50 = <50 μM) | Moderately active (E50 = 50–100 μM) | Slightly active (E50 = >100 μM) | Not active (E50 = NEO) |
| 2,2′,4,4′ | 3,3′,4,4′ | DE-71 | DE-79 |
| 2,2′,4,4′,5 | 2,2′,4,4′,6 | ||
| 2,2′,4,4′,5,5′ | |||
E50 value indicates the effective concentration that increases the control activity by 50%. NEO = no effect observed up to 100 μM.
Not active up to 200 nM. The numbers represent the position of halogens on the phenyl rings. (Adapted from Kodavanti et al., Toxicol. Appl. Pharmacol. 130: 140–148, 1995).
The disruption of Ca2+-homeostasis may have a significant effect on other signal transduction pathways (e.g., inositol phosphate [IP] and AA second messengers) regulated or modulated by Ca2+. The congener 2,2′-DCB, but not 3,3′,4,4′,5-PeCB affected basal and carbachol (CB)-stimulated IP accumulation in cerebellar granule cells (Kodavanti et al., 1994). Further studies indicated that any modulation of CB-stimulated IP accumulation is due to Ca2+-overload, but not due to activation of PKC activity (Kodavanti et al., 1994; Shafer et al., 1996). AA is released intracellularly following activation of membrane phospholipases, and AA is an important second messenger in releasing Ca2+ from endoplasmic reticulum (Striggow and Ehrlich, 1997). Aroclor 1254 (a commercial mixture of PCBs) and 2,2′-DCB increased [3H]-AA release in cerebellar granule cells while 4,4-DCB did not (Kodavanti and Derr-Yellin, 1999); this is in agreement with previous structure-activity relationship studies on Ca2+ buffering and PKC translocation (Kodavanti and Tilson, 1997). A similar increase in [3H]-AA was observed with structurally similar chemicals such as PBDE mixtures (Kodavanti and Derr-Yellin, 2002). Further studies indicated that the [3H]-AA release caused by these chemicals could be due to activation of both Ca2+ dependent and -independent phospholipase A2 (PLA2).
One of the down-stream effects of perturbed Ca2+-homeostasis is translocation of PKC from the cytosol to the membrane where it is activated (Trilivas and Brown, 1989). [3H]-Phorbol ester ([3H]-PDBu) binding has been used as an indicator of PKC translocation. The congener 2,2′-DCB increased [3H]-PDBu binding in a concentration-dependent manner in cerebellar granule cells, while 3,3′,4,4′,5-PeCB had no effect with concentrations up to 100 μM. The effect of 2,2′-DCB was time-dependent, and also dependent on the presence of external Ca2+ in the medium. Sphingosine, a PKC translocation blocker, prevented 2,2′-DCB-induced increases in [3H]-PDBu binding (Kodavanti and Tilson, 2000). Experiments with several pharmacological agents revealed that the effects are additive with glutamate, and none of the channel (glutamate, calcium, and sodium) antagonists blocked the response of 2,2′-DCB (Kodavanti et al., 1994). Immunoblots of PKC-alpha and epsilon indicated that non-coplanar ortho-PCB decreased the cytosolic form and increased the membrane form significantly at 25 μM (Yang and Kodavanti, 2001).
These studies were further extended to understand the structure-activity relationship (SAR) of a number of PCB congeners and related chemicals. The reason for the selection of these chemicals is provided in Table 2. The results indicated several structural requirements to exhibit their activity in neuronal cells. Congeners with ortho-substitutions, especially together with lateral substitutions such as 2,2′,4,6-tetrachlorobiphenyl and 2,2′,4,6,6′-pentachlorobiphenyl, are most active (Tables 3 and 4). Congeners without ortho-substitutions such as 3,3′,4,4′-tetrachlorobiphenyl and 3,3′,4,4′,5-pentachlorobiphenyl, which assume more coplanar configurations tend not to be active (Tables 3 and 4). The data presented in Tables 3 and 4 clearly indicated that the potency of the PCB congeners in neurons is more directly associated with the structural configuration of PCB congeners than their hydrophobicity. The data also indicate that low lateral substitution, especially without para-substitution, or lateral content in the presence of ortho-substitution, may be the most important structural requirement for the in vitro activity of these congeners in neuronal preparations. SAR studies conclude that congeners that are noncoplanar increased PKC translocation while coplanar congeners did not (Tables 3 and 4; Kodavanti et al., 1995; Kodavanti and Tilson, 1997 and 2000; Fischer et al., 1998). This was further strengthened by the observations with structurally similar chemicals such as DDT, polychlorinated diphenyl ethers and polybrominated diphenyl ethers (Tables 3 and 4; Kodavanti et al., 1996b). Nitric oxide (NO), which is produced by NOS, is a gaseous neurotransmitter. NO has an important role as a retrograde messenger in LTP, learning and memory processes, and endocrine function (Schuman and Madison, 1994; McCann et al., 1998). The congener 2,2′-DCB, but not 4,4′-DCB, inhibited both cytosolic (nNOS) and membrane (eNOS) forms of NOS (Sharma and Kodavanti, 2002).
TABLE 3.
Comparative effects of persistent organic pollutants (POPs) in vitro on intracellular signaling (PKC translocation and microsomal Ca2+-buffering), neurotransmiiters (dopamine), and thyroid hormones (binding to transthyretin). Values shown are the mean concentrations, which caused a 50% change (increase or decrease). At these concentrations, there was no cytotoxicity. The numbers in parenthesis and bold indicate the relative potency against 2,2′, 5,5′-tetrachlorobiphenyl based on the effects on PKC translocation.
| POLYCHLORINATED BIPHENYL MIXTURES | |||||
|---|---|---|---|---|---|
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| Aroclor 1016 | 71 μM (0.394) | 6.8 μM | |||
| Arolcor 1254 | 56 μM (0.500) | 6.3 μM | |||
| Aroclor 1260 | >100 μM (0.280) | 7.6 μM | |||
| PCB CONGENERS | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| 2,2′ | 43 μM (0.651) | 8.0 μM | 64 μM | >1000 nM | |
| 3,3′ | 60 μM (0.467) | 12.5 μM | 195 μM | >1000 nM | |
| 3,5 | 74 μM (0.378) | 17.2 μM | 84 nM | ||
| 4,4′ | NEO | NEO | NEO | NEO | |
| 2,2′,6 | 58 μM (0.483) | 7.0 μM | 820 nM | ||
| 2,4,4′ | >100 μM (0.280) | 6.9 μM | 196 μM | 950 nM | |
| 2,3,4 | >201 μM | ||||
| 2,2′,4,4′ | 89 μM (0.315) | 5.8 μM | 115 μM | 918 nM | |
| 2,2′,4,4′ | 38 μM (0.737) | ||||
| 2,2′,4,6 | 41 μM (0.683) | 7.3 μM | 71 μM | ||
| 2,2′,4,6′ | 50 μM (0.560) | 2.4 μM | |||
| 2,2′,5,5′ | 28 μM (1.000) | 4.9 μM | 86 μM | 699 nM | |
| 2,2′,5,6′ | NEO | ||||
| 2,2′,6,6′ | NEO | NEO | NEO | NEO | |
| 2,4,4′,5 | |||||
| 3,3′,4,4′ | NEO | NEO | NEO | NEO | |
| 3,3′,5,5′ | 72 μM (0.389) | >100 μM | 7 nM | ||
| 2,2′,3,5′,6 | 97 nM | ||||
| 2,2′,4,4′,5 | 244 nM | ||||
| 2,2′,4,4′,6 | 158 μM | 256 nM | |||
| 2,2′,4,5,5′ | 243 nM | ||||
| 2,2′,4,6,6′ | 38 μM (0.737) | 5.5 μM | 93 μM | >1000 nM | |
| 2,3,3′,4,4′ | 95 μM (0.295) | 5.3 μM | >1000 nM | ||
| 2,3,3′,4′,6 | 19 nM | ||||
| 2,3′,4,4′,5 | >100 μM (0.280) | 6.6 μM | >1000 nM | ||
| 3,3′,4,4′,5 | NEO | >100 μM | NEO | >1000 nM | |
| 3,3′,4,5,5′ | NEO | 6 nM | |||
| 2,2′,3,3′,4,4′ | >100 μM (0.280) | 4.9 μM | NEO | ||
| 2,2′,3,3′,5,5′ | >100 μM (0.280) | 5.1 μM | >1000 nM | ||
| 2,2′,3,3′,6,6′ | 58 μM (0.483) | 6.3 μM | >1000 nM | ||
| 2,2′,4,4′,5,5′ | >100 μM (0.280) | 6.6 μM | 90 nM | ||
| 2,3,3′,4,4′,5 | >100 μM (0.280) | 5.4 μM | >1000 nM | ||
| 3,3′,4,4′,5,5′ | NEO | NEO | 43 nM | ||
| 2,2′,3,4,4′,5,5′ | NEO | 4.8 μM | 690 nM | ||
| 2,2′,3,3′,5,6,6′ | |||||
| HYDROXY-PCBS | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| 2′,5′ | 70 μM (0.400) | 13.0 μM | |||
| 3,5 | NEO | 17.3 μM | |||
| 2,2′,5′ | 93 μM (0.301) | 4.6 μM | |||
| 2′,4′,6′ | 32 μM (0.875) | 3.9 μM | |||
| 3,4′,5 | NEO | 12.8 μM | |||
| 2,2′,4′,5,5′ | 80 μM (0.350) | 4.6 μM | |||
| 2,3,3′,4 | 8.8 nM | ||||
| 2,2′,3,3′,4 | 17 nM | ||||
| 2′,3,3′,4′,5 | 15 nM | ||||
| POLYCHLORINATED DIBENZOFURAN | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| 1,2,3,7,8 | NEO | NEO | |||
| POLYCHLORINATED DIBENZODIOXIN | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| 2,3,7,8 | NEO | NEO | |||
| POLYCHLORINATED DIPHENYL ETHER MIXTURES, CONGENERS, AND DDT | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| Nitrofen | >100 μM (0.280) | 15.3 μM | |||
| o,p′-DDT | >100 μM (0.280) | 4.7 μM | |||
| p,p′-DDT | >100 μM (0.280) | 4.4 μM | |||
| Diphenyl ether | 93 μM (0.301) | 66.5 μM | |||
| 4,4′ | 85 μM (0.329) | 6.7 μM | |||
| 2,4,4′ | 43 μM (0.651) | 5.5 μM | |||
| 3,3′,4,4′ | >100 μM (0.280) | 5.1 μM | |||
| 2,2′4,4′,5 | >100 μM (0.280) | 4.7 μM | |||
| 2,3′,4,4′,5 | >100 μM (0.280) | 4.8 μM | |||
| POLYBROMINATED DIPHENYL ETHER MIXTURES AND CONGENERS | |||||
| Intracellular signaling | |||||
| POP | PKC translocation1,2 | Ca2+-buffering3 | Dopamine4 | Thyroid hormones5 | |
| DE-71 | >100 μM (0.280) | 12.4 μM | |||
| DE-79 | NEO | NEO | |||
| 2,2′,4,4′ | 37 μM (0.757) | ||||
| 3,3′,4,4′ | 50 μM (0.560) | ||||
| 2,2′,4,4′,5 | >100 μM (0.280) | ||||
| 2,2′,4,4′,6 | >100 μM (0.280) | ||||
| 2,2′,4,4′,5,5′ | >100 μM (0.280) | ||||
Chauhan et al., (2000). Blank spaces indicate that the data are not available. NEO = no effect observed up to 100 μM.
These in vitro studies clearly demonstrated that second messenger systems, involved in the development of the nervous system, LTP, and learning and memory, are sensitive targets for the ortho-substituted PCBs and related chemicals. PCBs (ortho-PCBs and commercial PCB mixtures) affected intracellular signaling events at low micromolar concentrations and shorter exposure periods, where cytotoxicity is not evident. These signaling pathways include calcium homeostasis and PKC translocation. The rise of intracellular free Ca2+ is slow, but steady following exposure. This free Ca2+ rise could be due to increased calcium influx, inhibited Ca2+ buffering mechanisms, and/or calcium release from intracellular stores by the products of membrane phospholipases. The increases in free Ca2+ levels may lead to the translocation of PKC. The coplanar non-ortho-PCBs have marginal effects on calcium homeostasis and no effects on PKC translocation. Literature reports indicate that at slightly higher concentrations, commercial PCB mixtures (Aroclors 1221 and 1254) have been known to alter neurite outgrowth in PC12 cells (Angus and Contreras, 1995) and hypothalamic cells (Gore et al., 2002). PCBs have also been shown to inhibit thyroid hormone-dependent extension of dendrites of cerebellar Purkinje cells (Kuroda, 2003). It also appears that ortho-substituted PCBs can kill cerebellar granule cells by altering membrane structure (Tan et al., 2004) and it is possible that this structural change could be secondary to changes in intracellular signaling by these chemicals.
In vivo effects of PCBs on intracellular signaling have been studied with a commercial PCB mixture, Aroclor 1254, following adult or developmental exposures. Following exposure to adult rats, PCB mixture produced changes in second messenger systems that are similar to those observed after in vitro exposures (Kodavanti et al., 1998a) and concentrations that altered second messenger systems in vitro are achievable in brain in vivo (Kodavanti et al., 1998b). Following developmental exposure (given orally from gestational day 6 through postnatal day 21), Arolcor 1254 did not alter maternal body weight or percent mortality but caused a small, transient decrease in body weight gain of offspring. Both calcium homeostasis and PKC activities were significantly affected following developmental exposure to Aroclor 1254 (Kodavanti et al., 2000). Developmental exposure to PCBs also caused significant hypothyroxinemia and age-dependent alterations in the translocation of PKC isozymes; the effects were significant at postnatal day (PND) 14 (Yang et al., 2003). Immunoblot analysis of PKC-alpha (α), -gamma (γ), and -epsilon (ε) from both cerebellum and hippocampus revealed that developmental exposure to Aroclor 1254 caused a significant decrease in cytosolic fraction and an increase in particulate fraction. For some isozymes, the ratio between the two fractions was increased in a dose-dependent manner. Thus, the patterns of subcellular distributions of PKC isoforms following a developmental PCB exposure were PKC isozyme- and developmental stage-specific (Yang et al., 2003). The changes in PKC and other second messengers were associated with changes in transcription factors such as Sp1 and NF-kB indicating changes in gene expression following developmental exposure to PCBs (Riyaz Basha et al., 2006). PCBs have been shown to suppress thyroid hormone-dependent gene expression (Iwasaki et al., 2002). Considering the significant role of PKC signaling in motor behavior, learning and memory (Murphy et al., 1987; Chen et al., 1997), it is suggested that altered subcellular distribution of PKC isoforms at critical periods of brain development may be associated with activation of transcription factors and subsequent gene expression, and may be a possible mechanism of PCB-induced neurotoxic effects. PKC-α, γ, and ε may be among the target molecules implicated in PCB-induced neurological impairments after developmental exposure.
Further studies focused on the structural consequences of changes in the intracellular signaling pathway following developmental PCB exposure. Detailed brain morphometric evaluation was performed by measuring neuronal branching and spine density. In utero and lactational exposure to PCBs affected normal dendritic development of Purkinje cells and CA1 pyramids in animals at postnatal day 22 (Mervis et al., 2002). The branching area was significantly smaller in the PCB-exposed rats. At adulthood, there was continued neurostructural disruption of the CA1 dendritic arbor, but the branching area of the Purkinje cells returned to normal levels. Developmental exposure to PCBs also resulted in a significantly smaller spine density in hippocampus, but not in cerebellum. This dysmorphic cytoarchitecture could be the structural basis for long-lasting neurocognitive deficits in PCB-exposed rats (Mervis et al., 2002).
Previously, Pruitt et al (1999) reported a reduced growth of intra- and infra-pyramidal mossy fibers following developmental exposure to PCBs. Nguon et al. (2005) also reported that perinatal exposure to PCBs differentially affected the development of cerebellum in male and female rats. Considering the importance of intracellular signaling in neuronal development (Kater and Mills, 1991), these studies indicate that developmental exposure to a PCB mixture resulted in altered cellular distribution of PKC isoforms, which can subsequently disrupt the normal maintenance of signal transduction in developing neurons. The perturbations in intracellular signaling events could lead to structural changes in the brain. These findings suggest that altered subcellular distribution of PKC isoforms may be a possible mode of action for PCB-induced neurotoxicity.
Dose-response effects of POPs on hormonal imbalance
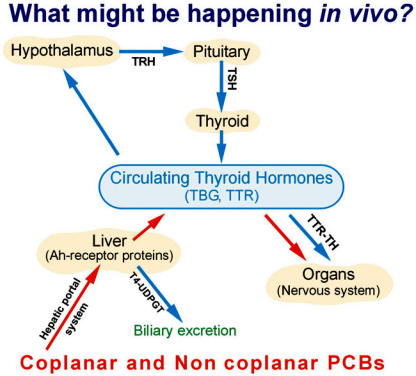
The function and development of the brain are influenced by hormones. Thyroid hormones (TH) have profound effects on neurological function in people of all ages. TH increase the rate of neuronal proliferation and stimulate differentiation (Lauder, 1977). Once neurons are formed, they follow an orderly pattern of migration to the appropriate areas in the brain. TH deficiency has been shown to cause disorganization of brain (Nicholson and Actman, 1972; Nunez, 1984). TH also stimulate formation and development of neuronal processes, both axons and dendrites (Legrand et al., 1982; Nunez, 1984; Kimura-Kuroda et al., 2002). TH are synthesized as mostly T4 (thyroxine) and small amounts of T3 (3,3′,5-triiodothyronine) in the thyroid gland by the uptake of iodide from the blood and are stored as amino acid residues of thyroglobulin. The T4 and T3 are released into circulation for transport to different organs. TH synthesis and secretion are regulated by a sensitive feedback mechanism by hypothalamic-pituitary axis (Figure 2). In the circulating human blood, T4 and T3 bind to the proteins such as thyroxine-binding globulin (TBG), transthyretin (TTR), and albumin with decreasing order of affinity. However, the high affinity TBG is not present in rodents, birds, amphibians or fish (Capen, 1992). Although TTR is not the major T4 transport protein in humans, TTR is thought to be essential for T4 transport to brain in humans (Herbert et al., 1986). In addition, TTR is the only TH binding plasma protein that is synthesized by both in liver and brain. TTR is reported to serve a role in mediating the delivery of T4 across the blood-brain barrier and the maternal to fetal transport through placenta (Schreiber et al., 1995; Southwell et al., 1993). TTR also plays an essential role in the determination of free T4 levels in the extra-cellular compartment of the brain, which is independent of T4 homeostasis in the body (Schreiber et al., 1995). Free TH in circulation is excreted through UDP glucuronidation (Figure 2). Once circulating T4 reaches different organs of the body, they are deiodinated by deiodinases releasing T3. Type I deiodinase is prevalent in liver while type II deiodinase is prevalent in brain (Visser et al., 1982; Kaplan et al., 1983). These hormones bind to the nuclear TH receptors to elicit their functional role.
FIGURE 2.
Disruption of thyroid hormone homeostais by polychlorinated biphenyls and the possible mechanisms. The solid arrow in blue shows the normal process for TH production and clearance. The solid arrow in red shows the predominant pathway for the effects of PCBs on TH homeostasis. The binding of PCBs to transport proteins in blood not only results in the reduction of circulating TH levels, but also gets transported to the organs to elicit an adverse effect. TRH, Thyrotropin-releasing hormone; TSH, Thyroid-stimulating hormone; TBG, Thyroxine-binding globulin; TTR, Transthyretin; T4, Thyroxine; UDPGT, UDP-glucuronyltransferase.
Many environmental contaminants including POPs alter TH function, either inhibiting the thyroidal system or mimicking it. Among the pollutants, PCBs and dioxins have dramatic effects on TH function in humans (Koopman-Esseboom et al., 1994; Langer et al., 1998; Nagayama et al., 1998), wildlife species (Brouwer et al., 1989; Van den Berg et al., 1994) and in experimental animals (Morse et al., 1993; Goldey et al., 1995; Zoeller et al., 2002). PCBs have been shown to decrease circulating TH levels during development (Morse et al., 1993; Crofton et al., 2000) and in adult animals (Porterfield, 1994; Kodavanti et al., 1998a). PCBs have also shown to decrease thyroid hormones in rat brain and stimulate type II deiodinase activity in brain (Morse et al., 1996b). Many studies have been performed to elucidate the mechanism(s) involved in POP-induced alterations in TH levels. In general, the decrease in circulating TH levels by chemicals could be at least by 3 known mechanisms. First, the chemical can directly affect the thyroid gland to decrease the synthesis of TH (Collins and Capen, 1980). Second, reduced TH levels can be caused by enhanced biliary excretion of T4 due to the induction of UDP-glucuronyltransferases (UDPGT; Van Birgelen et al., 1995). Third, the chemical may displace natural ligand, T4, binding to TTR (Lans et al., 1993; Morse et al., 1996b; Chauhan et al., 2000). Although all these three mechanisms might be responsible for the decreases in circulating TH by POPs (parent compound and metabolites), high affinity binding of POPs to TTR might be the key factor in eliciting the biological effects. Binding of POPs to TTR has been hypothesized to result in (1) a selective retention of POPs in plasma, (2) facilitated transport of the POPs over the placenta to the fetal compartment, and (3) decreased maternal and fetal plasma T4 levels by competition with the natural ligand, T4 (reviewed by Brouwer et al., 1998). In addition, POPs will be reaching the target organ such as brain by facilitated transport where POPs can bind to the target sites and elicit biological effect.
Studies by Chauhan et al. (2000) indicated the importance of ortho-substitution on PCB-binding to TTR. Hydroxy-PCBs have high affinity to TTR compared to the parent PCBs. The PCB congeners with di-meta substitution in one or both rings, which most closely resembling the diiodophenolic ring of T4, showed the highest TTR binding activity. PCB congener patterns with single meta substitution in one or both rings, which more closely resembling the monoiodophenolic ring of T3 showed significantly lower TTR binding activity. The binding potencies of several ortho-PCBs seem to follow the biological activities such as decreases in PC12-cellular dopamine content and PKC translocation in neuronal cells (Table 3). Among the PCBs, differential effects on circulating TH were observed based on the coplanarity. PCB congeners that are non-coplanar such as 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) decreased circulating T4 levels to a greater extent (>90%) than coplanar PCBs such as 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) (Craft et al., 2002). However, PCB 126 has high binding affinity towards TTR compared to PCB 153 (Chauhan et al., 2000). As shown in the Figure 2, both coplanar and noncoplanar PCBs enter the hepatic portal system through gastro-intestinal absorption from the food. Since liver has high content of Ah-receptor proteins, coplanar PCBs bind to these proteins with high affinity and only small amount returns to the circulation. On the other hand, most of the noncoplanar PCBs return to circulation since they have very low or no affinity for the Ah-receptor proteins. Although coplanar PCBs have strong affinity for TTR, the concentrations in the target organs could be very low due to their sequestration in liver. In support of this, Saghir et al (2000) reported that the accumulation of 14C-PCB169 in liver was 3-fold higher compared to 14C-PCB153 and serum levels of 14C-PCB153 were about twice as high as those of 14C-PCB169. Accumulation of 14C-PCB153 in brain was 4- to 9-fold higher than that of 14C-PCB 169. Recent evidence also suggests that binding of PCBs to TTR might play a key role in decreasing circulating TH. Meerts et al. (2000) reported that perinatal exposure to hydroxylated PCB 107 (4-hydroxy 2,3,3′,4′,5-pentachlorobiphenyl) resulted in 89% decrease in total T4 levels in the absence of any increase in hepatic T4-UDP glucuronyltransferase or type I deiodinase activity. They have also demonstrated that the 14C-labeled PCB was bound to TTR in both maternal and fetal plasma. Hallgren and Darnerud (2002) reported that T4 decreases is mainly due to disturbances in serum transport, caused by binding of in vivo formed PCB and PBDE metabolites. Further, Kuriyama et al. (2003) reported exposure to PCB118 during development resulted in decreased circulating T4 levels by 42% at weaning without any changes in liver weight gain or induction of EROD activity. In other studies, microsomal inducers including phenobarbital, PCB, 3-methylcholanthrine, and pregnenolone-16α-carbonitrile caused induction of T4-UDP glucuronidation to a similar extent while similar doses caused decreases in serum T4 levels to a different extent (Hood et al., 2003) suggesting that mechanisms other than T4-UDP glucuronidation are responsible for decreases in circulating T4 levels. These studies led to insight that binding of PCBs to TTR may play an important role in PCB-induced decreases in circulating thyroid hormones and that the neurotoxic consequences following exposure to these chemicals may not be dependent on Ah-receptor activation. Even though in humans thyroxine binding globulin is the main TH transport protein in the blood, TTR still plays a role in mediating the delivery of T4 across the blood-brain barrier, transporting T4 into the cerebrospinal fluid and transferring maternal T4 to the fetal through placenta (Calvo et al., 1990; Southwell et al., 1993). In order to exert biological effect, TH should bind to the thyroid hormone receptors (TR) in the nucleus. Although some in vitro studies showed interference of PCBs with TR thereby affecting TH-sensitive genes (Bogazzi et al., 2003) and TR-mediated transcription (Miyazaki et al., 2004), other in vivo studies showed that PCBs exert TH-like effects in the fetal brain but do not bind to TR (Gauger et al., 2003). On the other hand, Kumura-Kuroda et al. (2005) reported that hydroxy metabolites of PCBs inhibit TH-dependent extension of cerebellar Purkinje cell dendrites. Additional studies are needed to confirm the role of TH in PCB-induced neurotoxicity.
Table 3 shows the concentrations, which altered neurochemical parameters by 50% of control activity. It is obvious that there is a good correlation among the effects of PCB congeners on all three neurochemical endpoints. For PKC translocation, 2,2′,5,5′-tetrachlorobiphenyl seems to be the most active congener. Using this congener as a base, the relative potencies of other PCB congeners and structurally related chemicals were calculated (Table 3). Since the effects of PCBs on intracellular signaling, neurotransmitters, and thyroid hormone homeostasis occurred at biologically and environmentally relevant concentrations, these potential mechanisms might be involved in the neurotoxic effects of PCBs. The reasons are: (1) PCBs affected intracellular signaling, neurotransmitters, and thyroid hormone homeostasis at low micromolar concentrations; (2) Low micromolar concentrations accumulated in rat brain regions; (3) intracellular signaling, neurotransmitters, and thyroid hormone homeostasis are crucial for nervous system development and neuronal function; (4) PCBs affected neuronal development in vitro and in vivo, and caused learning and memory deficits in humans and animals.
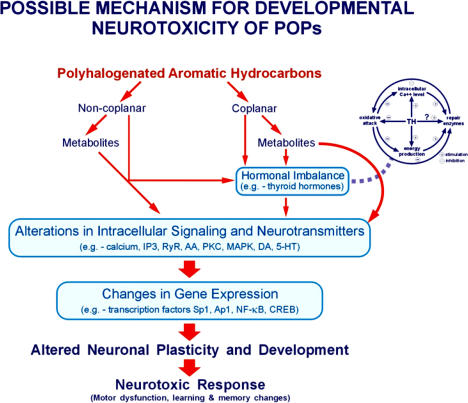
In addition to the mechanisms mentioned above, PCBs have been shown to alter the cholinergic system (Juarez de Ku et al., 1994) and cause oxidative stress (Mariussen et al., 2002; Lee and Opanashuk, 2004). It has also been demonstrated that inhibitors of nitric oxide synthase and phospholipase A2 led to a significant reduction of oxidative stress and cell death (Mariussen et al., 2002). Although studies on these systems are limited, both of them have a significant role in the function of the nervous system. Additional studies are needed to test whether one or more of these mechanisms are responsible for the effects of PCBs in the nervous system. Figure 3 shows the proposed mode of action for the neurotoxic consequences following exposure to PCBs. It is obvious from Tables 3 and 4 that polyhalogenated aromatic hydrocarbons (PAHs) can be separated as non-coplanar and coplanar PAHs. Both parent compounds and their hydroxy metabolites have differential effects on the three proposed mechanisms. Non-coplanar parent compounds affected intracellular signalling, thyroid hormone homeostasis, and neurotransmitters. The effects on intracellular signalling include calcium homeostasis, inositol phosphate and ryanodine receptors, membrane phosholipases, and protein kinases. The neurotransmitters include dopamine and serotonin. The hydroxy metabolites of PCBs affected intracellular signalling endpoints with equal potency, but affected thyroid hormone homeostasis to a greater extent (Table 3). On the other hand, coplanar parent compounds have effects only on thyroid hormones but no effects on neurotransmitters and intracellular signaling. The coplanar metabolites have been shown to affect intracellular signaling and thyroid hormones. In the case of non-coplanar PAHs, all three proposed mechanisms might be working together to cause neurotoxic response while coplanar PAHs could be working through one or two of the proposed mechanisms to cause neurotoxic response (Figure 3). In any case, the changes in these proposed pathways could lead to changes in the gene expression of several transcription factors (e.g., Sp1, Ap1, and NF-kB) that may lead to altered neuronal plasticity and development, and finally lead to a neurotoxic response such as motor dysfunction or learning and memory deficits.
FIGURE 3.
Possible mechanism(s) for developmental neurotoxicity of persistent organic pollutants (e.g., PCBs). The polyhalogenated aromatic hydrocarbons (PAHs) can be separated as non-coplanar and coplanar compounds. Non-coplanar parent compounds and hydroxy metabolites affected intracellular signalling, thyroid hormone (TH) homeostasis, and neurotransmitters. On the other hand, coplanar parent compounds have effects only on TH while no effects on neurotransmitters and intracellular signaling. The coplanar metabolites have been shown to affect intracellular signaling and TH. In the case of non-coplanar PAHs, all three proposed mechanisms might be working together to cause neurotoxic response while coplanar PAHs could be working through one or two of the proposed mechanisms to cause neurotoxic response. The changes in these proposed pathways could lead to changes in the gene expression of several transcription factors that may lead to altered neuronal plasticity and development, and finally lead to a neurotoxic response such as motor dysfunction or learning and memory deficits.
FURTHER CONSIDERATIONS
Recently, there has been growing recognition of an apparent increase in the incidence of developmental disabilities. About 17% of children under 18 years old (nearly 12 million) in the USA suffer from deafness, speech deficits, cerebral palsy, delay in growth and development, emotional or behavioral problems, or learning disabilities (Boyle et al., 1994) and this number is increasing (CDC, 2003). Improved reporting and differing diagnostic criteria may explain some but not all of the trends (Schettler, 2001). Although genetic factors have a key role in these developmental disabilities, environmental chemicals may also be causative factors for various developmental disorders of the brain, such as Attention Deficient Hyperactive Disorder (ADHD) and/or autism.
Developmental neurotoxicity involves alterations in behavior, neurophysiology, neurochemistry, and/or gross dysmorphology of the nervous system occurring in the offspring as a result of chemical exposure in utero and during lactation. Compared to adults, the developing organism has its own physiologic characteristics, uptake characteristics, and possible inherent susceptibilities. The developing nervous system may be uniquely sensitive to toxicants, since there is rapid growth of the brain, known as “brain growth spurt”. In rodents, this period is entirely neonatal (the first 3–4 postnatal weeks). In humans, the “brain growth spurt” begins during the third trimester of pregnancy and continues throughout the first years of life. During this period, the brain goes through several developmental processes starting from neurogenesis to proliferation, migration, and differentiation. Chemical exposure during any of these processes could have detrimental effects on the establishment of normal brain structure, current brain functioning and indirectly on subsequent brain functioning in adulthood. Intracellular signaling pathways are crucial for the normal function and development of the nervous system. The intracellular second messengers generated in this process offer key steps in the mode of action for developmental neurotoxicity of a number of chemicals including PCBs (see Figure 1 for other structurally related chemicals) and provide good scientific basis for more quantitative and biologically defensible human health risk assessment.
Polybrominated diphenyl ethers (PBDEs) are good examples where information obtained from structurally related chemicals such as PCBs could be applied. PBDEs are used as flame-retardants in textiles, electrical equipment, plastics, and building materials. PBDEs are structurally similar to PCBs with ten substitutions for halogens and 209 possible congeners based on position and number of halogens (Figure 1). All PBDE congeners are non-coplanar in nature because of the structural configuration. Based on SAR results with PCBs, one can predict that PBDEs should be active in the nervous system. In agreement with this prediction, PBDEs have been shown to affect signal transduction pathways (Kodavanti and Derr-Yellin, 2002; Kodavanti, 2003; Kodavanti and Ward, 2005; Kodavanti et al., 2005; Reistad and Mariussen, 2005), alter circulating thyroid hormones (Hallgren et al., 2001), and cause learning and memory deficits in mice and rats (Eriksson et al., 2001; Viberg et al., 2004; Dufault et al., 2005). Although human evidence for the developmental neurotoxicity of PBDEs is not available, there is considerable public health concern since PBDEs have been detected in human blood, adipose tissue, and breast milk and the levels are rapidly rising in wildlife, humans, and the environment (Gill et al., 2004). Due to the continued use of PBDEs in consumer products and their bioaccumulative nature, attention must be paid for the potential health risks associated with exposure to these and other structurally related chemicals (McDonald, 2005). PBDEs have similar effects on the nervous system like PCBs and other structurally related chemicals (Eriksson et al., 2001; Kodavanti and Derr-Yellin, 2002; Kodavanti, 2003). PCBs and PBDEs have been shown exert neurobehavioral effects (Eriksson et al., 2001) and cause changes in intracellular signaling pathways in neuronal cells (Kodavanti et al., 2004) at equimolar doses/concentrations suggesting a common mode of action for these chemicals. Considering the structural similarities of PBDEs and PCBs and the known health effects of PCBs in humans, these two groups of chemicals (may be other structurally related chemicals) could conceivably work through the same mechanism(s), to cause developmental neurotoxicity.
ACKNOWLEDGMENTS
The author is grateful for the graphic assistance of Mr. John Havel and for the editorial comments of Mr. Tom Ward of USEPA, Dr. Frode Fonnum of Norwegian Research Establishment, Dr. Jae-Ho Yang of Catholic University of Daegu, Dr. Nigel Walker of NIEHS, and Dr. Wayne Bowers of Health Canada on an earlier version of this manuscript. This manuscript was reviewed by the NHEERL, U.S. Environmental Protection Agency, and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The opinions expressed by the authors are not to be misconstrued as U.S. Environmental Protection Agency policy.
REFERENCES
- Agrawal A.K., Tilson H.A., Bondy S.C. 3,4,3′,4′-Tetrachlorobiphenyl given to mice prenatally produces long-term decreases in striatal dopamine and receptor binding sites in the caudate nucleus. Toxicol. Lett. 1981;7:417–424. doi: 10.1016/0378-4274(81)90087-4. [DOI] [PubMed] [Google Scholar]
- Angus W.G., Contreras M.L. Aroclor 1254 alters the binding of 125I-labeled nerve growth factor in PC12 cells. Neurosci. Lett. 1995;191:23–26. doi: 10.1016/0304-3940(94)11547-x. [DOI] [PubMed] [Google Scholar]
- ATSDR (1999). Toxicological profile for polychlorinated bipheyls, prepared by Research Triangle Institute, ATSDR document, Atlanta, GA.
- Bemis J.C., Seegal R.F. Polychlorinated biphenyls and methylmercury alter intracellular calcium concentrations in rat cerebellar granule cells. Neurotoxicology. 2000;21:1123–1134. [PubMed] [Google Scholar]
- Bemis J.C., Seegal R.F. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Bogazzi F., Raggi F., Ultimieri F., Russo D., Campomori A., McKinney J.D., Pinchera A., Bartalena L., Martino E. Effects of a mixture of polychlorinated bipheyls (Aroclor 1254) on the transcriptional activity of thyroid hormone receptor. J. Endocrinol. Invest. 2003;26:972–978. doi: 10.1007/BF03348194. [DOI] [PubMed] [Google Scholar]
- Bowman R.E., Heironimus M.P., Barsotti D.A. Locomotor hyperactivity in PCB-exposed rhesus monkeys. Neurotoxicology. 1981;2:251–268. [PubMed] [Google Scholar]
- Boyle C., Decoulfle P., Yeargin-Allsopp M. Prevalence and health impact of developmental disabilities in US children. Pediatrics. 1994;93:399–403. [PubMed] [Google Scholar]
- Brouwer A., Morse D.C., Lans M.C., Schuur A.G., Murk A.J., Klasson-Wehler E., Bergman A., Visser T.J. Interactions of persistent environmental organohalogens with the thyroid hormone system: Mechanisms and possible consequences for animal and human health. Toxicol. Ind. Hlth. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Brouwer A., Reijnders P.J.H., Koeman J.H. Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A and thyroid hormone deficiency in the common seal (Phoca vitulina) Aquatic Toxicol. 1989;15:99–106. [Google Scholar]
- Bushnell P.J., Moser V.C., MacPhail R.C., Oshiro W.M., Derr-Yellin E.C., Phillips P.M., Kodavanti P.R.S. Neurobehavioral assessments of rats perinatally exposed to a commercial mixture of polychlorinated biphenyls. Toxicol. Sci. 2002;68:109–120. doi: 10.1093/toxsci/68.1.109. [DOI] [PubMed] [Google Scholar]
- Calvo R., Obregon M.J., Ruij de Ona C., Escobar del Rey F., Morreale de Escobar G. Congenital hypothyroidism, as studied in rats. The crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J. Clin. Invest. 1990;86:889–899. doi: 10.1172/JCI114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capen C.C. Pathophysiology of chemical injury of the thyroid gland. Toxicol. Lett. 1992;64/65:381–388. doi: 10.1016/0378-4274(92)90211-2. [DOI] [PubMed] [Google Scholar]
- Carpenter D.O., Stoner C.R., Lawrence D.A. Flow cytometric measurements of neuronal death triggered by PCBs. Neurotoxicology. 1997;18:507–513. [PubMed] [Google Scholar]
- Centers for Disease Control. (2003). Summary of health statistics for the US population: National heath interview survey 2001. Vital and Health Statistics, Series 10, Number 217. [PubMed]
- Chauhan K.R., Kodavanti P.R.S., McKinney J.D. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol. Appl. Pharmacol. 2000;162:10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Hsu C.C. Effects of prenatal exposure to PCBs on the neurological function of children: a neuropsychological and neurophysiological study. Dev. Med. Child Neurol. 1994;36:312–320. doi: 10.1111/j.1469-8749.1994.tb11851.x. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Yu M.L., Rogan W.J., Gladen B.C., Hsu C.C. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am. J. Public Health. 1994;84:415–421. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.J., Sweatt J.D., Klann E. Enhanced phosphorylation of the postsynaptic protein kinase C substrate RC3/neurogranin during long-term potentiation. Brain Res. 1997;749:181–187. doi: 10.1016/s0006-8993(96)01159-6. [DOI] [PubMed] [Google Scholar]
- Choksi N.Y., Kodavanti P.R.S., Tilson H.A., Booth R.G. Effects of polychlorinated biphenyls (PCBs) on brain tyrosine hydroxylase activity and dopamine synthesis in rats. Fundam. Appl. Toxicol. 1997;39:76–80. doi: 10.1006/faat.1997.2351. [DOI] [PubMed] [Google Scholar]
- Chou S.M., Miike T., Payne W.M., Davis G.J. Neuropathology of “spinning syndrome” induced by prenatal intoxication with a PCB in mice. Ann. N. Y. Acad. Sci. 1979;320:373–395. doi: 10.1111/j.1749-6632.1979.tb56619.x. [DOI] [PubMed] [Google Scholar]
- Collins W.T., Jr., Capen C.C. Fine structural lesions and hormonal alterations in thyroid glands of perinatal rats exposed in utero and by the milk to polychlorinated biphenyls. Am. J. Pathol. 1980;99:125–142. [PMC free article] [PubMed] [Google Scholar]
- Costa L.G. Ontogeny of second messenger systems. In: Slikker W.L., Chang L.W., editors. Handbook of Developmental Neurotoxicology. San Diego, CA: Academic Press; 1998. pp. 275–284. [Google Scholar]
- Craft E.S., DeVito M.J., Crofton K.M. Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57BL/6J mice exposed to TCDD-like and Phenobarbital-like polychlorinated biphenyl congeners. Toxicol. Sci. 2002;68:372–380. doi: 10.1093/toxsci/68.2.372. [DOI] [PubMed] [Google Scholar]
- Crofton K.M., Kodavanti P.R.S., Derr-Yellin E.C., Casey A.C., Kehn L.S. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol. Sci. 2000;57:131–140. doi: 10.1093/toxsci/57.1.131. [DOI] [PubMed] [Google Scholar]
- Dufault C., Poles G., Driscoll L.L. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol. Sci. 2005;88:172–180. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Erickson M.D. Analytical Chemistry of PCBs. Boston: Butterworth; 1986. [Google Scholar]
- Eriksson P., Fredriksson A. Developmental neurotoxicity of four ortho-substituted polychlorinated biphenyls in the neonatal mouse. Environ. Toxicol. Pharmacol. 1996;1:155–165. doi: 10.1016/1382-6689(96)00015-4. [DOI] [PubMed] [Google Scholar]
- Eriksson P., Jakobsson E., Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.H. Free radicals in brain metabolism and pathology. Br. Med. Bull. 1993;49:577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- Fischer L.J., Seegal R.F., Ganey P.E., Pessah I.N., Kodavanti P.R.S. Symposium overview: Toxicity of non-coplanar PCBs. Toxicol. Sci. 1998;41:49–61. doi: 10.1006/toxs.1997.2386. [DOI] [PubMed] [Google Scholar]
- Gauger K.J., Kato Y., Haraguchi K., Lehmler H-J., Robertson L.W., Bansal R., Zoeller R.T. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Perspect. 2003;112:516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A.M., Oshiro W.M., Haykal-Coates N., Kodavanti P.R.S., Bushnell P.J. Gender-dependent behavioral and sensory effects of a commercial mixture of polychlorinated biphenyls (Aroclor 1254) in rats. Toxicol. Sci. 2001;59:268–277. doi: 10.1093/toxsci/59.2.268. [DOI] [PubMed] [Google Scholar]
- Gilbert M.E., Mundy W.R., Crofton K.M. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol. Sci. 2000;57:102–111. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- Gill U., Chu I., Ryan J.J., Feeley M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev. Environ. Contam. Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- Girard P.R., Kuo J.F. Protein kinase C and its 80-kilodalton substrate protein in neuroblastoma cell neurite outgrowth. J. Neurochem. 1990;54:300–306. doi: 10.1111/j.1471-4159.1990.tb13315.x. [DOI] [PubMed] [Google Scholar]
- Goldey E.S., Kehn L.S., Crofton K.M. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Gore A.C., Wu T.J., Oung T., Lee J.B., Woller M.J. A novel mechanism for endocrine-disrupting effects of polychlorinated biphenyls: direct effects on gonadotropin-releasing hormone neurons. J. Neuroendocrinol. 2002;14:814–823. doi: 10.1046/j.1365-2826.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., Burse V.W., Needham L.L., Storr-Hansen E., Heinzow B., Debes F., Murata K., Simonsen H., Ellefsen P., Budtz-Jorgensen E., Keiding N., White R.F. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol. Teratol. 2001;23:305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Hallgren S., Sinjari T., Hakansson H., Darnerud P.O. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch. Toxicol. 2001;75:200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- Hallgren S., Darnerud P.O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphneyls (PCBs) and chlorinated paraffins (CPs) in rats – testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Harada M. Intrauterine poisoning: clinical and epidemiological studies and significance of the problem. Bull Inst Constitutional Med (Suppl) 1976;25:1–60. [Google Scholar]
- Herbert J., Wilcox J.N., Pham K.T.C., Fremeau R.T., Zeviani M., Dwork A., Soprano D.R., Makover A., Goodman D.S., Zimmerman E.A., Roberts J.L., Schon E.A. Transthyretin: a choroid plexus-specific transport protein in human brain. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- Herr D.W., Graff J.E., Derr-Yellin E.C., Crofton K.M., Kodavanti P.R.S. Flash-, somatosensory-, and peripheral nerve-evoked potentials in rats perinatally exposed to Aroclor 1254. Neurotoxicol. Teratol. 2001;23:591–601. doi: 10.1016/s0892-0362(01)00180-5. [DOI] [PubMed] [Google Scholar]
- Holene E., Nafstad I., Skaare J.U., Bernhoft A., Engen P., Sagvolden T. Behavioral effects of pre- and postnatal exposure to individual polychlorinated biphenyl congeners in rats. Environ. Toxicol. Chem. 1995;14:967–976. [Google Scholar]
- Holene E., Nafstad I., Skaare J.U., Sagvolden T. Behavioral hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav. Brain Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- Hood A., Allen M.L., Liu Y., Liu J., Klaassen C.D. Induction of T4-UDP-GT activity, serum thyroid stimulating hormone, and thyroid follicular cell proliferation in mice treated with microsomal enzyme inducers. Toxicol. Appl. Pharmacol. 2003;188:6–13. doi: 10.1016/s0041-008x(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Inglefield J.R., Shafer T.J. Polychlorinated biphenyl-stimulation of Ca2+ oscillations in developing neocortical cells: a role for excitatory transmitters and L-type voltage-sensitive Ca2+ channels. J. Pharmacol. Exp. Ther. 2000;295:105–113. [PubMed] [Google Scholar]
- Inglefield J.R., Mundy W.R., Meacham C.A., Shafer T.J. Identification of calcium-dependent and –independent signalling pathways involved in polychlorinated biphenyl-induced cyclic AMP-responsive element-binding protein phosphorylation in developing cortical neurons. Neurosci. 2002;115:559–573. doi: 10.1016/s0306-4522(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Miyazaki W., Takeshita A., Kuroda Y., Koibuchi N. Polychlorinated biphenyls (PCBs) suppress thyroid hormone-induced transactivation. Biochem. Biophys. Res. Comm. 2002;299:384–388. doi: 10.1016/s0006-291x(02)02659-1. [DOI] [PubMed] [Google Scholar]
- Jacobson J.L., Jacobson S.W, Humphrey H.E.B. Effects of in utero exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol. Teratol. 1990;12:319–326. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- Jacobson J.L., Jacobson S.W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. The New Eng. J. Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Juarez de Ku L.M., Sharma-Stokkermans M., Meserve L.A. Thyroxine normalizes polychlorinated biphenyl (PCBP dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day old rats. Toxicology. 1994;94:19–30. doi: 10.1016/0300-483x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Kafafi S.A., Afeefy H.Y., Ali A.H., Said H.K., Kafafi A.G. Binding of polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ. Hlth. Persp. 1993;101:422–428. doi: 10.1289/ehp.93101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.M., Visser T.J., Yaskoski K.A., Leonard J.L. Characteristics of iodothyronine tyrosyl ring deiodination by rat cerebral cortical microsomes. Endocrinology. 1983;112:35–42. doi: 10.1210/endo-112-1-35. [DOI] [PubMed] [Google Scholar]
- Kater S.B., Millls L.R. Regulation of growth cone behavior by calcium. J. Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J., Nagata I., Kuroda Y. Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Dev. Brain Res. 2005;154:259–263. doi: 10.1016/j.devbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on intracellular signaling in rat neuronal cultures. Organohal. Comp. 2003;65:1–4. [Google Scholar]
- Kodavanti P.R.S. Intracellular signaling and developmental neurotoxicity. In: Zawia N.H., editor. Molecular Neurotoxicology: Environmental Agents and Transcription-Transduction Coupling. Boca Raton, FL: CRC Press; 2004. pp. 151–182. [Google Scholar]
- Kodavanti P.R.S., Derr-Yellin E.C. Activation of calcium-dependent and –independent phospholipase A2 by non-coplanar polychlorinated biphenyls in rat cerebellar granule neurons. Organohalogen Comp. 1999;42:449–453. [Google Scholar]
- Kodavanti P.R.S., Derr-Yellin E.C. Differential effects of polybrominated diphenyl ethers and polychlorinated bipheyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol. Sci. 2002;68:451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Tilson H.A. Structure-activity relationships of potentially neurotoxic PCB congeners in the rat. NeuroToxicology. 1997;18:425–442. [PubMed] [Google Scholar]
- Kodavanti P.R.S., Tilson H.A. Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways. Ann. N.Y. Acad. Sci. 2000;919:97–105. doi: 10.1111/j.1749-6632.2000.tb06872.x. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Ward T.R. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signalling in rat brain in vitro. Toxicol. Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Shin D., Tilson H.A., Harry G.J. Comparative effects of two polychlorinated biphenyl congeners on calcium homeostasis in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 1993;123:97–106. doi: 10.1006/taap.1993.1226. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Shafer T.J., Ward T.R., Mundy W.R., Freudenrich T., Harry G.J., Tilson H.A. Differential effects of polychlorinated biphenyl congeners on phosphoinositide hydrolysis and protein kinase C translocation in rat cerebellar granule cells. Brain Res. 1994;662:75–82. doi: 10.1016/0006-8993(94)90797-8. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Ward T.R., McKinney J.D., Tilson H.A. Increased [3H]-phorbol ester binding in rat cerebellar granule cells by polychlorinated biphenyl mixtures and congeners: structure-activity relationships. Toxicol. Appl. Pharmacol. 1995;130:140–148. doi: 10.1006/taap.1995.1018. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Ward T.R., McKinney J.D., Tilson H.A. Inhibition of microsomal and mitochondrial Ca2+ sequestration in rat cerebellum by polychlorinated biphenyl mixtures and congeners: structure-activity relationships. Arch. Toxicol. 1996;70:150–157. doi: 10.1007/s002040050254. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Ward T.R., McKinney J.D., Waller C.L., Tilson H.A. Increased [3H]phorbol ester binding in rat cerebellar granule cells and inhibition of 45Ca2+ sequestration by polychlorinated diphenyl ether congeners and analogs: structure-activity relationships. Toxicol. Appl. Pharmacol. 1996;138:251–261. doi: 10.1006/taap.1996.0123. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Derr-Yellin E.C., Mundy W.R., Shafer T.J., Herr D.W., Barone S., Jr., Choksi N.Y., MacPhail R.C., Tilson H.A. Repeated exposure of adult rats to Aroclor 1254 causes brain region-specific changes in intracellular Ca2+ buffering and protein kinase C activity in the absence of changes in tyrosine hydroxylase. Toxicol. Appl. Pharmacol. 1998;153:186–198. doi: 10.1006/taap.1998.8533. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Ward T.R., Derr-Yellin E.C., Mundy W.R., Casey A.C., Bush B., Tilson H.A. Congener-specific distribution of polychlorinated biphenyls in brain regions, blood, liver, and fat of adult rats following repeated exposure to Aroclor 1254. Toxicol. Appl. Pharmacol. 1998;153:199–210. doi: 10.1006/taap.1998.8534. [DOI] [PubMed] [Google Scholar]
- Kodavanti P.R.S., Mundy W.R., Derr-Yellin E.C., Tilson H.A. Developmental exposure to Aroclor 1254 alters calcium buffering and protein kinase C activity in the brain. Toxicol. Sci. 2000;54(supplement):76–77. [Google Scholar]
- Kodavanti P.R.S., Ward T.R., Ludewig G., Robertson L., Birnbaum L. Polybrominated dipheyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol. Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C., Morese D.C., Weisglas-Kuperus N., Lutke-Schipholt I.J., Van der Paauw C.G., Tuinstra L.G., Brouwer A., Sauer P.J. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr. Res. 1994;36:468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J., Nagata I., Negishi-Kato M., Kuroda Y. Thyroid hormone-dependent development of mouse cerebellar Purkinje cells in vitro. Dev. Brain Res. 2002;137:55–65. doi: 10.1016/s0165-3806(02)00408-x. [DOI] [PubMed] [Google Scholar]
- Kuratsune M., Yoshimara T., Matsuzaka J., Yamaguchi A. Yusho, a poisoning caused by rice oil contaminated with polychlorinated biphenyls. HSMHA Health Rep. 1971;86:1083–1091. [PMC free article] [PubMed] [Google Scholar]
- Kuratsune M., Yoshimara T., Matsuzaka J., Yamaguchi A. Epidemiological study on Yusho: A poisoning caused by ingestion of rice oil contaminated with a commercial brand of polychlorinated bipheyls. Enviorn. Health Perspect. 1972;1:119–128. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S., Fidalgo-Neto A., Mathar W., Palavinskas R., Friedrich K., Chahoud I. Effect of low dose mono-ortho 2,3′,4,4′,5-pentachlorobiphenyl on thyroid hormone status and EROD activity in rat offspring: consequences for risk assessment. Toxicology. 2003;186:11–20. doi: 10.1016/s0300-483x(02)00602-9. [DOI] [PubMed] [Google Scholar]
- Kuroda Y. Effects of endocrine disruptors on brain development – development of novel assay systems for risk assessment. Environ. Sci. 2003;10(suppl):023–033. [Google Scholar]
- Lang V. Polychlorinated biphenyls in the environment. J. Chrom. 1992;595:1–43. doi: 10.1016/0021-9673(92)85144-i. [DOI] [PubMed] [Google Scholar]
- Langer P., Tajtakova M., Fodor G., Kocan A., Bohov P., Michalek J., Kreze A. Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated bipheyls. Eur. J. Endocrinol. 1998;139:402–409. doi: 10.1530/eje.0.1390402. [DOI] [PubMed] [Google Scholar]
- Lans M.C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorinated biphenyls, -dibenzo-p-dioxins, and –dibenzofurans with human transthyretin. Chem. Biol. Interact. 1993;88:7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- Lauder J.M. Effects of thyroid state on development of rat cerebellar cortex. In: Grave GD, editor. Thyroid Hormone and Brain Development. New York: Raven Press; 1977. pp. 235–254. [Google Scholar]
- Lee D.W., Opanashuk L.A. Polychlorinated biphenyl mixture Aroclor 1254-induced oxidative stress plays a major role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Legrand C., Clos J., Legrand J. Influence of altered thyroid and nutritional states on early histogenesis of the rat cerebellar cortex with special reference to synaptogenesis. Reprod. Nutr. Dev. 1982;22(Suppl 1B):201–208. doi: 10.1051/rnd:19820206. [DOI] [PubMed] [Google Scholar]
- Limke T.L., Otero-Montanez J.K., Atchison W.D. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J. Pharmacol. Exp. Ther. 2003;304:949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Longnecker M.P., Wolff M.S., Gladen B.C., Brock J.W., Grandjean P., Jacobson J.L., Korrick S.A., Rogan W.J., et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M.A. Analysis of the mechanisms underlying the age-related impairment in long-term potentiation in the rat. Rev. Neurosci. 1998;9:169–201. doi: 10.1515/revneuro.1998.9.3.169. [DOI] [PubMed] [Google Scholar]
- Mariussen E., Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Mariussen E., Morch-Anderson J., Fonnum S. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol. Appl. Pharmacol. 1999;161:274–282. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- Mariussen E., Myhre O., Reistad T., Fonnum F. The polychlorinated biphenyl mixture Aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol. Appl. Pharmacol. 2002;179:137–144. doi: 10.1006/taap.2002.9353. [DOI] [PubMed] [Google Scholar]
- McCann S.M., Kimura M., Walczewska A., Karanth S., Rettori V., Yu W.H. Hypothalamic control of FSH and LH by FSH-RF, LHRH, cytokines, leptin and nitric oxide. Neuroimmunomodulation. 1998;5:193–202. doi: 10.1159/000026337. [DOI] [PubMed] [Google Scholar]
- McDonald T.A. Polybrominated diphenyl ether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integ. Environ. Assess. Management. 2005;1:343–354. [PubMed] [Google Scholar]
- McKinney J.D., Waller C.L. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Hlth. Perspect. 1994;102:290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts I.A.T.M., Assink Y., Cenijn P.H., van den Berg J.H.J., Weijers B.M., Bergman A., Koeman J.H., Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol. Sci. 2000;68:361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Mervis R.F., Bachstetter A.D., Harry G.J., Tilson H.A., Kodavanti P.S. Long-lasting neurostructural consequences in the rat hippocampus by developmental exposure to a mixture of polychlorinated biphenyls. Toxicol. Sci. 2002;66(1-S):133. [Google Scholar]
- Miyazaki W., Iwasaki T., Takeshita A., Kuroda Y., Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J. Biol. Chem. 2004;279:18195–18202. doi: 10.1074/jbc.M310531200. [DOI] [PubMed] [Google Scholar]
- Morse D.C., Groen D., Veerman M., Van Amerongen C.J., Koeter H.B.W.M., Smits Van Prooije A.E., Visser T.J., Koeman J.H., Brouwer A. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol. Appl. Pharmacol. 1993;122:27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Morse D.C., Seegal R.F., Brosch K.O., Brouwer A. Long-term alterations in regional brain serotonin metabolism following maternal polychlorinated biphenyl exposure in the rat. Neurotoxicology. 1996;17:631–638. [PubMed] [Google Scholar]
- Morse D.C., Wehler E.K., Wesseling W., Koeman J.H., Brouwer A. Alterations in rat brain thyroid hormone status following pre- and post-natal exposure to polychlorinated biphenyls (Aroclor 1254) Toxicol. Appl. Pharmacol. 1996;136:269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Mundy W.R., Shafer T.J., Tilson H.A., Kodavanti P.R.S. Extracellular calcium is required for the polychlorinated biphenyl-induced increase of intracellular free calcium levels in cerebellar granule cell culture. Toxicology. 1999;136:27–39. doi: 10.1016/s0300-483x(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Murphy S., McCabe N., Morrow C., Pearce B. Phorbol ester stimulates proliferation of astrocytes in primary cultures. Brain Res. 1987;428:133–135. doi: 10.1016/0165-3806(87)90091-5. [DOI] [PubMed] [Google Scholar]
- Nagayama J., Okamura K., Iida T., Hirakawa H., Matsueda T., Tsuji H., Hasegawa M., Sato K., Ma H.-Y., Yanagawa T., Igarashi H., Fukushige J., Watanabe T. Postnatal exposure to chlorinated dioxins and related chemicals on thyroid hormone status in Japanese breast-fed infants. Chemosphere. 1998;37:1789–1793. doi: 10.1016/s0045-6535(98)00244-6. [DOI] [PubMed] [Google Scholar]
- Nguon K., Baxter M.G., Sajdel-sulkowska E.M. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female rat neonates. The Cerebellum. 2005;4:112–122. doi: 10.1080/14734220510007860. [DOI] [PubMed] [Google Scholar]
- Nicholson J.L., Actman J. The effects of early hypo- and hyperthyroidism on the development of rat cerebellar cortex. 1. cell proliferation and differentiation. Brain Res. 1972;44:13–23. doi: 10.1016/0006-8993(72)90362-9. [DOI] [PubMed] [Google Scholar]
- Niemi W.D., Audi J., Bush B., Carpenter D.O. PCBs reduce long-term potentiation in the CA1 region of rat hippocampus. Exp. Neurol. 1998;151:26–34. doi: 10.1006/exnr.1998.6793. [DOI] [PubMed] [Google Scholar]
- Nihei M.K., McGlothan J.L., Toscano C.D., Guilarte T.R. Low level Pb2+ exposure affects hippocampal protein kinase-gamma gene and protein expression in rats. Neurosci. Lett. 2001;298:212–216. doi: 10.1016/s0304-3940(00)01741-9. [DOI] [PubMed] [Google Scholar]
- Nishida N., Farmer J.D., Kodavanti P.R.S., Tilson H.A., MacPhail R.C. Effects of acute and repeated exposures to Aroclor 1254 in adult rats: motor activity and flavor aversion conditioning. Fundam. Appl. Toxicol. 1997;40:68–74. doi: 10.1006/faat.1997.2352. [DOI] [PubMed] [Google Scholar]
- Nunez J. Effects of thyroid hormones during brain differentiation. Mol. Cell Endocrinol. 1984;37:125–132. doi: 10.1016/0303-7207(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Okey A.B., Riddick D.S., Harper P.A. The Ah receptor: Mediator of the toxicity of 2,3,7,8-tetrachlorodibezo-p-dioxin (TCDD) and related compounds. Toxicol. Lett. 1994;70:1–22. doi: 10.1016/0378-4274(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Pantaleoni G., Fannini D., Sponta A.M., Palumbo G., Giorgi R., Adams P. Effects of maternal exposure to PCBs on F1 generation behavior in the rat. Fundam. Appl. Toxicol. 1988;11:440–449. doi: 10.1016/0272-0590(88)90108-x. [DOI] [PubMed] [Google Scholar]
- Patandin S., Lanting C.I., Mulder P.G.H., Boersma E.R., Sauer P.J.J., Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediat. 1999;134:33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- Porterfield S. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ. Health Perspect. 1994;102(Suppl 2):125–130. doi: 10.1289/ehp.94102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt D.L., Meserve L.A., Bingman V.P. Reduced growth of intra- and infra-pyramidal mossy fibers is produced by continuous exposure to polychlorinated biphenyl. Toxicology. 1999;138:11–17. doi: 10.1016/s0300-483x(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Reistad T., Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (DE-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol. Sci. 2005;87:57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Richardson J.R., Miller G.W. Acute exposure to Aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol. Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Riyaz Basha M., Braddy S., Zawia N.H., Kodavanti P.R.S. Ontogenetic alterations in prototypic transcription factors in the rat cerebellum and hippocampus following perinatal exposure to a commercial PCB mixture. Neurotoxicology. 2006;27:118–124. doi: 10.1016/j.neuro.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Roegge C.S., Seo B-W., Crofton K.M., Schantz S.L. Gestational-lactational exposure to Aroclor 1254 impairs radial-arm maze performance in male rats. Toxicol. Sci. 2000;57:121–130. doi: 10.1093/toxsci/57.1.121. [DOI] [PubMed] [Google Scholar]
- Rogan W.J., Gladen B.C., Hung K-L., et al. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science. 1988;241:334–336. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- Rogan W.J., Gladen B.C. Neurotoxicology of PCBs and related compounds. Neurotoxicology. 1992;13:27–36. [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. CRC Crit. Rev. Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Saghir S.A., Hansen L.G., Holmes K.R., Kodavanti P.R.S. Differential and non-uniform tissue and brain distribution of two distint 14C-hexachlorobiphenyls in weanling rats. Toxicol. Sci. 2000;54:60–70. doi: 10.1093/toxsci/54.1.60. [DOI] [PubMed] [Google Scholar]
- Schantz S.L., Levin E.D., Bowman R.E., Heironimus M.P., Laughlin N.K. Effects of perinatal PCB exposure on discrimination-reversal learning in monkeys. Neurotoxicol. Teratol. 1989;11:243–250. doi: 10.1016/0892-0362(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Schantz S.L. Developmental neurotoxicity of PCBs in humans: What do we know and where do we go from here. Neurotoxicol. Teratol. 1996;18:217–227. doi: 10.1016/s0892-0362(96)90001-x. [DOI] [PubMed] [Google Scholar]
- Schantz S.L., Moshtaghian J., Ness D.K. Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam. Appl. Toxicol. 1995;26:117–126. doi: 10.1006/faat.1995.1081. [DOI] [PubMed] [Google Scholar]
- Schettler T. Toxic threat to neurologic development of children. Environ. Health Perspect. 2001;109:813–816. doi: 10.1289/ehp.01109s6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Southwell B.R., Richardson S.J. Hormone delivery system to the brain – transthyretin. Exp. Clin. Endocrinol. Diabetes. 1995;103:75–80. doi: 10.1055/s-0029-1211332. [DOI] [PubMed] [Google Scholar]
- Schuman E.M., Madison D.V. Nitric oxide and synaptic function. Annu. Rev. Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Seegal R.F. The neurochemical effects of PCB exposure are age-dependent. Arch. Toxicol. 1994;16:128–137. doi: 10.1007/978-3-642-78640-2_15. [DOI] [PubMed] [Google Scholar]
- Seegal R.F. Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit. Rev. Toxicol. 1996;26:709–737. doi: 10.3109/10408449609037481. [DOI] [PubMed] [Google Scholar]
- Seegal R.F., Brosch K.O., Bush B. Polychlorinated biphenyls produce regional alterations of dopamine metabolism in rat brain. Toxicol. Lett. 1986;30:197–202. doi: 10.1016/0378-4274(86)90103-7. [DOI] [PubMed] [Google Scholar]
- Seegal R.F., Bush B., Brosch K.O. Sub-chronic exposure of rat to Aroclor 1254 selectively alters central dopaminergic function. Neurotoxicology. 1991;12:55–66. [PubMed] [Google Scholar]
- Seegal R.F., Brosch K.O., Okoniewski R.J. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol. Appl. Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- Seegal R.F., Schantz S.L. Neurochemical and behavioral sequelae of exposure to dioxins and PCBs. In: Schecter A., editor. Dioxins and Health. New York: Plenum press; 1994. pp. 409–447. [Google Scholar]
- Shafer T.J., Mundy W.R., Tilson H.A., Kodavanti P.R.S. Disruption of inositol phosphate accumulation in cerebellar granule cells by polychlorinated biphenyls: a consequence of altered Ca2+ homeostasis. Toxicol. Appl. Pharmacol. 1996;141:448–455. doi: 10.1006/taap.1996.0311. [DOI] [PubMed] [Google Scholar]
- Shain W., Bush B., Seegal R.F. Neurotoxicity of PCBs: Structure-activity relationships of individual congeners. Toxicol. Appl. Pharmacol. 1991;111:33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Sharma R., Kodavanti P.R.S. In vitro effects of polychlorinated biphenyls and hydroxy metabolites on nitric oxide synthases in rat brain. Toxicol. Appl. Pharmacol. 2002;178:127–136. doi: 10.1006/taap.2001.9328. [DOI] [PubMed] [Google Scholar]
- Sharma R., Derr-Yellin E.C., House D.E., Kodavanti P.R.S. Age-dependent effects of Aroclor 1254 on calcium uptake by subcellular organelles in selected brain regions of rats. Toxicology. 2000;156:13–25. doi: 10.1016/s0300-483x(00)00328-0. [DOI] [PubMed] [Google Scholar]
- Sinks T., Steele G., Smith A.B., Watkins K., Shults R.A. Mortality among workers exposed to polychlorinated biphenyls. Am. J. Epidemiol. 1992;136:389–398. doi: 10.1093/oxfordjournals.aje.a116511. [DOI] [PubMed] [Google Scholar]
- Southwell B.R., Duan W., Alcorn D., Brack C., Richardson S.J., Kohrle J., Schreiber G. Thyroxine transport to the brain: Role of protein synthesis by the choroid plexus. Endocrinology. 1993;133:2116–2126. doi: 10.1210/endo.133.5.8404661. [DOI] [PubMed] [Google Scholar]
- Storm J.E., Hart J.L., Smith R.F. Behavior of mice after pre- and postnatal exposure to Aroclor 1254. Neurobehav. Toxicol. Teratol. 1981;3:5–9. [Google Scholar]
- Striggow F., Ehrlich B.E. Regulation of intracellular calcium release channel function by arachidonic acid and leukotriene B4. Biochem. Biophys. Res. Comm. 1997;237:413–418. doi: 10.1006/bbrc.1997.7152. [DOI] [PubMed] [Google Scholar]
- Swedish Environmental Protection Agency (SEPA). (1998). Persistent Organic Pollutants: a Swedish view of an international problem. Monitor 16.
- Tan Y., Chen C-H., Lawrence D., Carpenter D.O. Ortho-substituted PCBs kill cells by altering membrane structure. Toxicol. Sci. 2004;80:54–59. doi: 10.1093/toxsci/kfh119. [DOI] [PubMed] [Google Scholar]
- Tilson H.A., Jacobson J.L., Rogan W.J. Polychlorinated biphenyls and the developing nervous system: cross-species comparisons. Neurotoxicol. Teratol. 1990;12:239–248. doi: 10.1016/0892-0362(90)90095-t. [DOI] [PubMed] [Google Scholar]
- Tilson H.A., Kodavanti P.R.S. Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology. 1997;18:727–744. [PubMed] [Google Scholar]
- Tilson H.A., Kodavanti P.R.S. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19:517–526. [PubMed] [Google Scholar]
- Trilivas I., Brown J.H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J. Biol. Chem. 1989;264:3102–3107. [PubMed] [Google Scholar]
- Van Birgelen A.P.J.M., Smit E.A., Kampen I.M., Groeneveld C.N., Fase K.M., Van der Kolk J., Poiger H., Van den Berg M., Koeman J.H., Brouwer A. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur. J. Pharmacol. 1995;293:77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Van den Berg M., Cranne B.L.H.J., Sinnige T., Van Mourik S., Dirksen S., Boudewijn T., Van der Gaag M., Lutke-Schipholt I.J., Spenkelink A., Brouwer A. Biochemical and toxic effects of polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in the cormorant (Phalacrocorax carbo) after in ovo exposure. Environ. Toxicol. Chem. 1994;13:803–816. [Google Scholar]
- Viberg H., Fredriksson A., Eriksson P. Investigations of strain and/or gender differences in developmental neurotoxic effects of polybrominated diphenyl ethers in mice. Toxicol. Sci. 2004;81:344–353. doi: 10.1093/toxsci/kfh215. [DOI] [PubMed] [Google Scholar]
- Visser T.J., Leonard J.L., Kaplan M.M., Larsen P.R. Kinetic evidence suggesting two mechanisms for iodothyronine 5′-deiodination in rat cerebral cortex. Proc. Natl. Acad. Sci. USA. 1982;79:5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voie O.A, Fonnum F. Ortho-substituted polychlorinated biphenyls elevate intracellular [Ca2+] in human granulocytes. Environ. Toxicol. Pharmacol. 1998;5:105–112. doi: 10.1016/s1382-6689(97)10064-3. [DOI] [PubMed] [Google Scholar]
- Walkowiak J., Wiener J.A., Fastabend A., Heinzow B., Kramer U., Schmidt E., Steingruber H.J., Wundram S., Winneke G. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet. 2001;358:1602–1607. doi: 10.1016/S0140-6736(01)06654-5. [DOI] [PubMed] [Google Scholar]
- Winneke G., Bucholski A., Heinzow B., Kramer U., Schmidt E., Walkowiak J., Wiener J.A., Steingruber H.J. Developmental neurotoxicity of polychlorinated biphenyls (PCBs): cognitive and psychomotor functions in 7-month old children. Toxicol. Lett. 1998;102–103:423–428. doi: 10.1016/s0378-4274(98)00334-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1993). Environmental Health Criteria 140: Polychlorinated Biphenyls and Terphenyls, 2nd ed. Prepared by S. Dobson and G.J. van Esch. International Programme on Chemical Safety, Geneva, Switzerland.
- Yamashita F, Hayashi M. Fetal PCB syndrome: clinical features, intrauterine growth retardation and possible alteration in calcium metabolism. Environ. Hlth. Perspect. 1985;59:41–45. doi: 10.1289/ehp.59-1568075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-H., Kodavanti P.R.S. Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells. Biochem. Biophys. Res. Comm. 2001;280:1372–1377. doi: 10.1006/bbrc.2001.4283. [DOI] [PubMed] [Google Scholar]
- Yang J-H., Derr-Yellin E.C., Kodavanti P.R.S. Alterations in brain protein kinase C isoforms following developmental exposure to a polychlorinated biphenyl mixture. Mol. Brain Res. 2003;111:123–135. doi: 10.1016/s0169-328x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- Zoeller R.T., Dowling A.L.S., Herzig C.T.A., Iannacone E.A., Gauger K.J., Bansal R. Thyroid hormone, brain development, and the environment. Environ. Health Perspect. 2002;110(suppl 3):355–361. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]