Abstract
Mediator proteins are required for transcriptional regulation of most genes in yeast. Mammalian Mediator homologs also function as transcriptional coactivators in vitro; however, their physiological role in gene-specific transcription is not yet known. To determine the role of Mediator proteins in the development of complex organisms, we purified putative Mediator complexes from Caenorhabditis elegans and analyzed their phenotypes in vivo. C. elegans Mediator homologs were assembled into two multiprotein complexes. RNA interference assays showed that the CeMed6, CeMed7, and CeMed10/CeNut2 gene products are required for the expression of developmentally regulated genes, but are dispensable for expression of the ubiquitously expressed genes tested in this study. Therefore, the gene-specific function of Mediator as an integrator of transcriptional regulatory signals is evolutionarily conserved and is essential for C. elegans development.
In eukaryotic transcription, the direct interaction of a gene-specific regulatory protein with the basal transcription machinery plays an important role in the transcriptional regulation of some genes (1, 2). However, for a number of eukaryotic genes, transcriptional coactivator proteins are required to achieve precisely orchestrated gene expression (3, 4). In particular, coactivators that are associated with the basal transcription machinery [for example, TATA-binding protein (TBP)-associated factors (TAFs) and Mediator complexes] are required to integrate diverse gene-specific regulatory signals at and to recruit basal transcription machinery to specific promoters. In addition, chromatin-remodeling complexes containing Swi2 homologs and/or histone modification proteins are required for activated transcription from a chromatin template (5–7).
Although coactivator proteins appear to be required for transcriptional regulation in general, functional characterizations of certain coactivators from higher eukaryotes have revealed unique and specific roles for these proteins in diverse developmental processes. For example, the TFIID complex is essential for transcriptional activation in a reconstituted system consisting of pure general transcription factors (8, 9). Depletion or inactivation of TFIID-specific TAFs (TAFII) from Saccharomyces cerevisiae affects the transcription of a subset of genes involved in cell cycle regulation (10, 11). The distinct requirement for TAFII also was observed in Drosophila; mutations in the TAFII110 and TAFII60 subunits affect Dorsal-mediated transcriptional activation when a reduced amount of the Dorsal gene product is present (12). The human and Drosophila counterparts of the yeast Swi/Snf complex facilitate the binding of transcriptional activator proteins to nucleosomal templates in vitro (13, 14), and mutations in Drosophila genes that encode subunits of these protein complexes affect cell proliferation (15) and peripheral nervous system development (16). On the other hand, CBP-1, a histone acetyltransferase gene from Caenorhabditis elegans, plays a critical role in promoting nonneuronal somatic differentiation (17). These distinct phenotypes of coactivator mutants suggest that individual coactivator proteins may regulate diverse, but specific developmental processes in higher eukaryotes.
Using activator-squelching assay (18), the Mediator complex was first identified in yeast as a distinct intermediary molecule that mediates signal transfer between gene-specific transcriptional activator proteins and the basal transcription machinery. The yeast Mediator complex is tightly associated with RNA polymerase II (pol II) and enables pol II to respond to transcriptional activators in an in vitro system reconstituted with pure general transcription factors (19). The yeast Mediator complex is composed of the Med proteins (Med1, Med2, Med4, Med6, Med8, Med9, Med10, and Med11), Gal11, Rgr1, Sin4, Hrs1, Rox3, and the Srb family of proteins (Srb2, Srb4, Srb5, Srb6, and Srb7) (for review, see ref. 3). These Mediator components assemble into several functional modules that regulate distinct groups of genes (20, 21).
The search for homologous Mediator complexes in mammalian systems led to the identification of a human Srb/Med-containing cofactor complex (SMCC) (22), a negative regulator of activated transcription (NAT) complex (23), and mouse and human Mediator complexes (24, 25). In addition, purification of mammalian cofactors required for transcriptional activation identified homologs of yeast Mediator proteins that are components of the vitamin D-receptor interacting protein (DRIP) complex (26), the activator-recruited cofactor (ARC) complex (27), and the cofactor required for SP1 activation (CRSP) (28). These complexes contain more than one yeast Mediator homolog and share several components, but their overall compositions are quite different from each other and from that of the yeast Mediator. Both SMCC and NAT negatively regulate TFIID-mediated transcriptional activation in vitro (22, 23), and SMCC also was shown to mediate transcriptional activation in TAF-depleted nuclear extracts (29). Therefore, these mammalian Mediator homologs function as both positive and negative regulators of transcription in vitro. However, experimental evidence confirming the physiological relevance of these Mediator complexes is lacking. In particular, the developmental, gene-specific requirements of individual yeast Mediator homologs have not yet been deciphered.
With the completion of the C. elegans genome project, several homologs of yeast Mediator genes were identified in the C. elegans sequence database. To determine the physiological functions of these Mediator homologs and to elucidate the underlying mechanism by which these Mediator homologs regulate transcription in higher eukaryotes, we purified the putative C. elegans Mediator complexes and examined their mutant phenotypes with the use of an RNA interference (RNAi) assay. We present evidence that these Mediator homologs are present in two large, multiprotein complexes that appear to work together to regulate transcription. In addition, we show that the C. elegans Mediator homologs are required for transcriptional activation of developmentally regulated genes and dispensable for the transcription of constitutively expressed genes. Our genetic and biochemical data thus suggest that the function of Mediator as a coactivator for gene-specific transcriptional regulation is evolutionarily conserved and plays a key regulatory role in development.
Materials and Methods
Nematode Strains.
The Bristol N2 nematode strain was used as wild type. The swIs1[pRF4 rol-6(su1006)+ ceh-13∷GFP] transgenic line (FR317) was a gift from F. Mueller (University of Fribourg, Switzerland). An integration line containing pRF4rol-6(su1006) + nhr-2∷GFP was obtained from A. Sluder (University of Georgia, Athens). A transgenic line (PD8152) Ex[pRF4rol-6(su1006)] that contains an extrachromosomal rps-5∷GFP was a gift of J. Fleenor and A. Fire (Carnegie Institution of Washington, Baltimore), and the MH1188 strain of the genotype unc-119(ed3); him-5; pTG96(sur-5∷GFP)+ pDP# MM016 (rescuing plasmid of unc-119) was obtained from M. Han (University of Colorado, Boulder, CO).
Plasmids and Antibodies.
Lambda phages containing a CeMED6 cDNA and a CeMED7 cDNA were obtained from Y. Kohara (National Institute of Genetics, Japan), and the cDNA inserts were popped out to obtain pCM6–1 and pCM7–1, respectively. CeMED10/CeNUT2 cDNA was amplified by using the PCR and the Barstead cDNA library with primers CM10F (5′-CCC GGA TCC ATA TGG TTC AGC AAC AGC AGC AAA GC-3′) and CM10R (5′-CCC CCA AGC TTT TAT GAT AAA TCG AGA TCT TCT CT-3′) and cloned into pBluescript KS(+) (Stratagene) to make pCM10–1. The CeSRB7 partial cDNA was cloned by PCR amplification of genomic DNA by using primers CS7F (5′-CCC GGA TCC ATA TGG CTG ATC GAA TGA CCC AAC TA-3′) and CS7R (5′-CCC CCA AGC TTC TCA ATA TTT TTA GCG GCT TTT GC-3′) and cloned into pBluescript SK(+) to make pCS7–1. The W03F9.5 ORF of C. elegans was identified as C. elegans TFIIB (CeTFIIB) on the basis of its sequence similarity to that of other species and was cloned by PCR amplification of genomic DNA by using primers TFIIB-3 (5′-AAT ACG ACT CAC TAT AGC GGC TCC AGT CCA GTG-3′) and TFIIB-4 (5′-ARR AAC CCT CAC TAA AGG ACG AAT TTC CCA AC-3′). All the clones generated were confirmed for the absence of a mutation by DNA sequencing. The CeMed7, CeMed10/CeNut2, and CeSrb7 cDNAs were cloned into pRSET B (Invitrogen), pGEX 5X-1, and pGEX3 (Pharmacia), respectively, to make recombinant proteins for Ab generation. Polyclonal antibodies to each of the recombinant proteins containing the CeMed7, CeMed10/CeNut2, and CeSrb7 sequences were raised in rats and affinity-purified as described by Harlow and Lane (30).
Purification of C. elegans Mediator Complexes.
For biochemical analyses, we obtained 15 g of C. elegans embryos. For embryo harvest, we cultured 1,000 87-mm plates of nematode embryos to the gravid adult stage, washed the plates lightly with M9, and harvested with spreaders the embryos that remained on the plates. C. elegans embryo nuclear extracts were prepared as described previously (31) with slight modifications. All purification steps were carried out at 4°C, and all buffers contained 1 mM DTT/10 μM PMSF/20 nM pepstatin A/6 nM leupeptin/20 μM bis-benzamidine unless otherwise specified. Embryo nuclear extracts (19.5 mg of protein) were adjusted to a potassium acetate concentration of 60 mM by dilution with buffer A0 (50 mM Hepes-KOH, pH 7.6/10% glycerol/1 mM EDTA) and applied to a 5-ml heparin-Sepharose column equilibrated with buffer A60 (buffers labeled as An are identical to buffer A0, except that they contain n mM potassium acetate). The column was washed with buffer A60, and bound proteins were eluted first with buffer A300 and then with A600. Superose-6 chromatography was conducted with the SMART system (Pharmacia) by using buffer B500 (buffer Bn is identical to buffer An except that it contains 0.1 mM EDTA and 0.2 mM 2-mercaptoethanol instead of 1 mM DTT) according to the manufacturer's instructions. Mediator proteins were monitored throughout the purification by immunoblot analysis.
Immunoprecipitation.
Anti-CeMed6 and anti-CeSrb7 Abs (100 μg) were conjugated with protein G-agarose (Sigma) beads (100 μl). The Ab beads (10 μl) were mixed with either nuclear extracts (50 μl; 157 μg proteins) or heparin-Sepharose fractions [200 μl; 45 μg of protein from the CeMed6 peak fraction (H3) and 23 μg of protein from the CeSrb7 peak fraction (H6)], and IP buffer (immunoprecipitation buffer; 20 mM Tris⋅Cl, pH 7.9/10% glycerol/100 mM potassium acetate/0.1% Nonidet P-40/0.1 mM EDTA) was added to a final volume of 300 μl. After incubating for 12 h at 4°C, the Ab beads were washed three times with 400 μl of IP buffer, and then the bound proteins were eluted twice with 20 μl of 0.1 glycine-HCl (pH 2.5), separated by SDS/PAGE, and monitored by immunoblotting.
Immunohistochemistry.
Embryos were permeabilized by freeze-cracking and fixed first in methanol (95%) and then in acetone for 5 min each at −20°C. Embryos were rehydrated in a 75%, 50%, and 25% methanol series (3 min at each step) and then washed in PBS three times for 10 min each. The blocking reaction was carried out in 5% goat serum for 3–5 h at room temperature. Anti-CeMed10/CeNut2 Abs (1:50), anti-CeMed6 Abs (1:50), and anti-CTD Abs (8WG16; 1:500) were added, and the embryos were incubated with the Abs overnight at 4°C. After washing in PBS three times each for 10 min, secondary Abs conjugated with rhodamine or FITC were added according to the manufacturer's instructions.
Double-Stranded RNAi Assay and Microscopy.
Standard microinjection procedures (32) were carried out. For the double-stranded RNA interference (dsRNAi), we injected either 20 or 200 μg/ml dsRNA corresponding to either CeMED6, CeMED7, or CeMED10/CeNut2 into wild-type N2 nematodes and/or four transgenic nematode lines that contained one of the following reporter genes: ceh-13∷GFP, nhr-2∷GFP, sur-5∷GFP, or rps-5∷GFP. As a control, we also injected CeTFIIB dsRNA (100 μg/ml) into the above transgenic nematode lines. In most experiments, the eggs were collected at intervals of 12 h, and their phenotypes were observed separately. Embryos were mounted on agar pads, and their phenotypes were observed with an Axioplan microscope (Zeiss).
Results and Discussion
The C. elegans Genome Has Genes Homologous to Several, But Not All, of the Yeast Mediator Component Genes.
To take advantage of the efficient gene knockout system of C. elegans for our developmental analysis of putative Mediator genes, we searched the C. elegans genome database for Mediator homologs. In addition to the CeMED6, CeMED10/CeNUT2, and CeRGR1 reported previously (28, 33, 34), we identified gene sequences YK166F10 and C24H11 as CeMED7 and CeSRB7, respectively (Fig. 1). CeMed7 (253 aa) is 16% identical to yeast (y) Med7 and 26% identical to human (h) Med7 throughout the entire ORF, whereas CeSrb7 (137 aa) showed only an 11% and 28% identity to ySrb7 and hSrb7, respectively. We were unable to identify sequences homologous to the rest of the yeast Mediator proteins in the C. elegans database. Therefore, homologs for yMed6, yMed7, yMed10/Nut2, ySrb7, and yRgr1 may constitute the key Mediator components that are evolutionarily conserved from yeast to human.
Figure 1.
Amino acid sequence alignment of Mediator homologs. Med7 (A) and Srb7 (B) in human, C. elegans, and yeast are compared. The conserved sequences are marked with boxes (the identical and biochemically similar amino acids are marked with black and lightly shaded boxes, respectively). Only the conserved regions are shown.
C. elegans Mediator Homologs Are Present in Multiprotein Complexes.
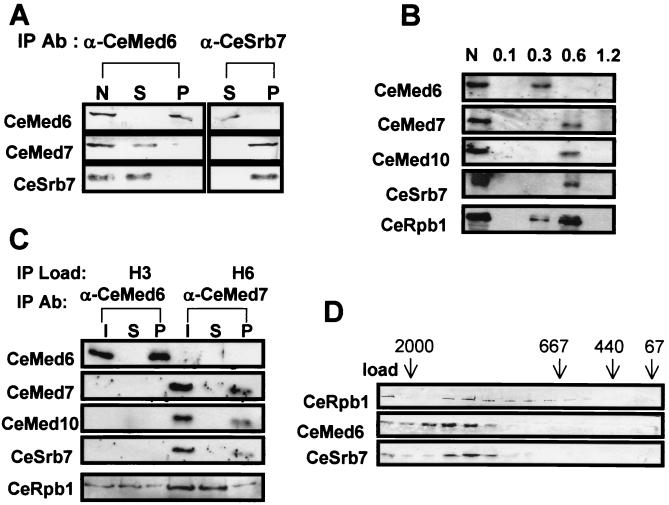
To examine whether the C. elegans Mediator homologs associate to form a complex as do their yeast counterparts, C. elegans embryo nuclear extract was immunoprecipitated with CeMed6 Ab and analyzed by immunoblotting. Affinity-purified CeMed6 Ab precipitated all of the CeMed6 protein from the extract, but contrary to our expectations, CeMed7 and CeSrb7 were not coprecipitated with CeMed6 (Fig. 2A). When we immunoprecipitated the nuclear extract with CeSrb7 Ab, CeMed7 coprecipitated with CeSrb7, but CeMed6 remained in the supernatant (Fig. 2A).
Figure 2.
Two distinct forms of C. elegans Mediator complexes. (A) Immunoprecipitation of C. elegans embryo nuclear extracts with the Mediator Abs indicated at the top of each lane. Equivalent amount of nuclear extract (N), supernatant (S), and pellet (P) of the immunoprecipitation were resolved on SDS/PAGE and immunoblotted with the Abs indicated at the left. (B) The heparin-Sepharose column fractions of C. elegans nuclear extract (N) eluted with the potassium acetate salt concentration (molar) indicated at the top of each lane were analyzed by SDS/PAGE and immunoblotting with the Abs indicated at the left. (C) CeMed6 and CeSrb7 complexes. Heparin fractions containing CeMed6 (H3) and CeSrb7 (H6) were immunoprecipitated with CeMed6 and CeSrb7 Abs, respectively, and the proteins that coprecipitated with CeMed6 or CeSrb7 were visualized by SDS/PAGE and immunoblot analysis with the Abs indicated at the left. (D) Superose-6 gel-filtration chromatography of C. elegans Mediator complexes. C. elegans nuclear extracts were fractionated on a Superose-6 column, and the fractions were subjected to SDS/PAGE and immunoblotted with the Abs indicated at the left. The migration positions of molecular size markers are indicated (kDa).
To rule out the possibility that the Abs used for the immunoprecipitation caused CeMed6 to dissociate from the other Mediator homologs, we examined whether CeMed6 is separable from CeSrb7 during a column fractionation of the embryo nuclear extracts. When we fractionated the extract with heparin-Sepharose chromatography, CeMed6 was eluted with 0.3 M potassium acetate (H3 fraction), whereas all of the other C. elegans Mediator homologs tested (CeSrb7, CeMed7, and CeMed10/CeNut2) coeluted at the higher salt concentration (0.6 M potassium acetate, H6) (Fig. 2B). When the H6 fraction was immunoprecipitated with anti-CeMed7 Ab, all of the CeMed7, CeSrb7, and CeMed10/CeNut2 coprecipitated with pol II (Fig. 2C). Similarly, all of the CeMed6 in the H3 fraction also was coimmunoprecipitated with pol II by anti-CeMed6 Ab. These results suggest that all of the C. elegans Mediator homologs are associated with pol II-interacting complexes. However, unlike in yeast, CeMed6 does not appear to form a stable complex with the other three C. elegans Mediator homologs tested.
To estimate the apparent size of the CeMed6- and CeSrb7-containing complexes, C. elegans nuclear extract was analyzed by Superose-6 gel-filtration chromatography. Both CeMed6 and CeSrb7 migrated together with pol II at a molecular size of 1.5 MDa (Fig. 2D). This result suggests that either two distinct complexes, each of which contains either CeMed6 or CeSrb7, or a complex that contains both of the Mediator homologs has a size comparable to the Mediator complexes of other species. In either case, the fact that multiple Mediator homologs reside in a large pol II-interacting complex strongly suggests that these C. elegans Mediator homologs are true functional homologs of the corresponding yeast and human Mediator proteins.
That CeMed6 was separated from the other Mediator homologs in the immunoprecipitation and column fractionation experiments is intriguing considering that the yeast Mediator is composed of two tightly associated subcomplexes (20). The yeast Rgr1 subcomplex is composed of yMed7, yMed10, ySrb7, and other positive and negative regulators of transcription, whereas the ySrb4 subcomplex contains yMed6 and other dominant Srb proteins that interact with the carboxyl-terminal domain of pol II (20). Therefore, the above results indicate that C. elegans Mediator may have a similar modular structure in which the CeMed6- and CeSrb7-containing subcomplexes are less tightly associated than are the comparable yeast Mediator subcomplexes. Identification of other C. elegans Mediator components would be needed to address this issue.
The CeMed6 and CeSrb7 Mediator Complexes Are Ubiquitously Expressed in the Nuclei.
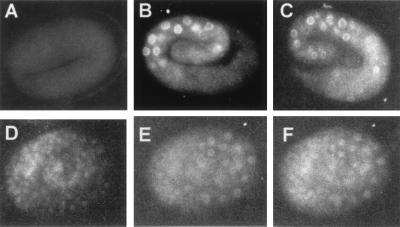
The biochemical separation of the C. elegans Mediator homologs suggests that these homologs may be required differentially during C. elegans development. However, immunohistochemical analysis of CeMed6 and CeMed10/CeNut2 in C. elegans embryos produced almost identical nuclei-staining patterns (Fig. 3 A–C). Both CeMed6 and CeMed10/CeNut2 proteins were expressed in the nuclei of all tissues examined throughout development. CeMed6 and CeMed10/CeNut2 proteins were found throughout the nucleoplasms, but appeared to be more densely localized in the periphery of the nuclei. When nuclei were double-stained with CeMed6 and pol II Abs, identical localization patterns for the two proteins were observed in all cells (Fig. 3 D–F). Because the distinct molecular functions of both yeast Mediator subcomplexes (the yMed6- and yMed10/Nut2-containing subcomplexes) are required together for transcriptional regulation (34), the colocalization pattern of the CeMed6 and CeMed10/CeNut2 proteins may suggest that both protein complexes work together in association with pol II to regulate transcription in most C. elegans cells.
Figure 3.
C. elegans Mediator complexes are expressed in all cell types and are localized in the nuclei. Wild-type embryos were stained with CeMed6 preimmune serum (A), CeMed6 Ab (B), or CeMed10/CeNut2 Ab (C). To compare the localization pattern of CeMed6 with that of pol II, wild-type embryos were stained with CeMed6 Ab (E) or pol II Ab (F), and the proteins were visualized by using indirect immunofluorescence. Embryos also were stained with 4′,6-diamidino-2-phenylindole (D) to show the location of nuclear DNA.
Mediator Homologs Are Essential for C. elegans Embryonic Development.
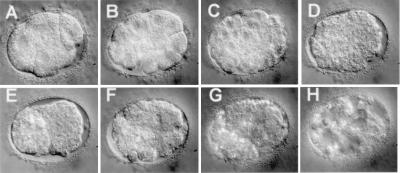
To assess the biological functions of the C. elegans Mediator gene products, we examined the mutant phenotypes of the Mediator homologs induced by dsRNAi (35). The dsRNAs corresponding to the coding sequences of CeMED6, CeMED7, and CeMED10/CeNUT2 were injected separately into the gonads of wild-type N2 nematodes at either high (200 ng/μl) or low (20 ng/μl) concentrations. When a high concentration of the dsRNAs was used, most of the progeny of the injected animals died at the embryonic stage (95–98% lethality) with an abnormally organized body pattern (Fig. 4). The embryos appeared to develop normally until the 300-cell stage (Fig. 4 A–E). However, in the following stages, the embryos developed abnormally, such that no apparent comma stages were discernible (Fig. 4F). The structures of the gut granules and pharynx were developed, but were not properly organized within the embryo (Fig. 4G). P granule staining showed that P granule formation and germ cell migration was not affected significantly (data not shown). A few hours later, the embryo began to disintegrate and died (Fig. 4H). When RNAi analyses were performed with low concentrations of either CeMED6, CeMED7, or CeMED10/CeNUT2 dsRNAs, a large percentage of the embryos developed to adulthood (62–94% for the embryos collected 0–12 h after dsRNA injection), but were sterile (Table 1). This result suggests that all of the Mediator genes we tested are required for germ-line development in adults as well as for embryogenesis.
Figure 4.
Embryonic lethality caused by RNAi of CeMED6. This series of photographs (A– H) shows embryos collected from animals injected with CeMED6 dsRNA at 0, 1, 3, 6, 8, 11, 14, and 33 h after transferring the injected animals to a slide glass. Embryo is shown at four-cell stage (A), 20-cell stage (B), ≈60-cell stage (C), and ≈300-cell stage (D). There are no visible abnormalities in the embryo up to this stage. However, these embryos never developed to the comma stage (E and F). (G) The pharynx and the gut granules were formed, and their movements were detected. (H) Internal cells of the embryo began to disintegrate, and no movement was observed. These results are representative of those observed in RNAi analysis of CeMED7 and CeMED10/CeNUT2.
Table 1.
RNAi analysis of C. elegans Mediator homologs
| RNAi* | Hours after injection† | Embryonic or larval lethal, % | Sterile adult, % | Normal adult, % | N |
|---|---|---|---|---|---|
| CeMED6 | 0–12 | 38 | 34 | 28 | 172 |
| 12–24 | 22 | 77 | 1 | 378 | |
| 24–40 | 89 | 10 | 1 | 381 | |
| CeMED7 | 0–12 | 8 | 51 | 41 | 187 |
| 12–24 | 23 | 20 | 57 | 450 | |
| 24–40 | 56 | 0 | 44 | 495 | |
| CeMED10 | 0–12 | 6 | 13 | 81 | 421 |
| 12–24 | 44 | 2 | 54 | 487 | |
| 24–40 | 84 | 0 | 16 | 744 |
N, total number of embryos examined.
*dsRNA (20 μg/ml) was injected into gonads of N2 wild-type nematodes.
†Embryo collection period after injection of dsRNA.
Despite the lack of apparent physical interaction between CeMed6 and the rest of the Mediator homologs, the fact that all three Mediator homologs are required for the same developmental processes in C. elegans once again suggests that both CeMed6- and CeSrb7 (and CeMed7)-containing complexes may work together for certain transcriptional activation. This regulatory mechanism in higher eukaryotes may increase the diversity of transcriptional regulation by using multiple Mediator protein modules and/or isoforms to build an intricate, multifunctional coactivator complex. Although we have identified only two forms of Mediator homolog-containing complexes, which appear to work together in C. elegans, additional Mediator isoforms may be present and functional during discreet developmental windows. In fact, a number of Mediator complexes [NAT (23), SMCC/TRAP (22), ARC (27), and CRSP (28)] that contain distinct sets of proteins have been identified in human cells.
C. elegans Mediator Complexes Are Required for Developmental Stage-Specific Transcriptional Regulation.
The embryonic lethal phenotypes caused by RNAi of several of the C. elegans Mediator homologs (Fig. 4 and Table 1) indicated that Mediator complexes are essential during early embryogenesis. To determine whether the C. elegans Mediator complexes function in the mediation of transcriptional regulatory signals, as do their yeast homologs, we examined the effect of Mediator and general transcription factor (CeTFIIB) mutations on the expression of C. elegans genes induced during embryogenesis. The C. elegans genes ceh-13 and nhr-2 contain promoters that are activated very early in embryogenesis, and their developmental stage-specific expression is important for embryonic development (36, 37). We analyzed the direct effects of Mediator RNAi on transcriptional activation of these genes by using transgenic nematode lines in which a green fluorescent protein (GFP) gene under the control of the ceh-13 or nhr-2 promoter (ceh-13∷GFP or nhr-2∷GFP) was integrated into the worm genome.
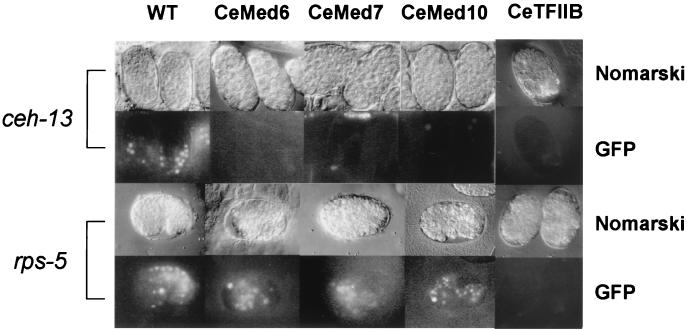
The embryos of ceh-13∷GFP-containing animals injected with buffer alone emitted strong fluorescence signals in a number of nuclei at early embryonic stages (Fig. 5). However, when either CeMED6, CeMED7, or CeMED10/CeNUT2 dsRNAs were injected into the ceh-13∷GFP-containing animals, the fluorescence signals were absent from their progeny at the same developmental stages (Fig. 5). Similar RNAi analyses with the nhr-2∷GFP transgenic animals yielded results that were identical to those obtained with the ceh-13∷GFP-containing animals (data not shown). Because the morphological defects of the RNAi mutants were not detectable until the comma stage of development, the lack of GFP expression in early-stage embryos did not result from reduced viability of the injected animals. Therefore, C. elegans Mediator genes are required for the transcriptional activation of developmental stage-specific inducible promoters from the ceh-13 and nhr-2 genes.
Figure 5.
Requirement for the C. elegans Mediators in transcriptional activation of developmental stage-specific genes in vivo. After microinjection of dsRNA corresponding to either CeMED6, CeMED7, CeMED10/CeNUT2, or CeTFIIB into transgenic nematode lines containing either the ceh-13∷GFP or rps-5∷GFP fusion genes, the embryos were observed for GFP fluorescence. Along the top, the dsRNAs used for the RNAi analysis are shown (WT, wild type, injected with buffer only). The promoters used to express GFP and the type of image [Nomarski or fluorescence (GFP)] are indicated at the left and right, respectively.
To examine whether Mediator complexes are also required for the transcription of constitutively expressed genes, we performed a similar experiment with two transgenic nematode lines containing GFP constructs under the control of ubiquitously expressed promoters from the sur-5 and rps-5 genes (ref. 38; A. Fire, personal communication). Because the transgenic nematode lines containing either the sur-5∷GFP or rps-5∷GFP fusion genes were not integration lines, only ≈57% (n = 218) and 39% (n = 49), respectively, of the embryos injected with buffer expressed GFP. Therefore, we measured the effect of RNAi on (i) the GFP fluorescence intensity in the embryos and (ii) the percentage of the cells expressing GFP. In contrast to the effect of RNAi on the expression of inducible gene promoters, injection of CeMED6, CeMED7, or CeMED10/CeNUT2 dsRNAs did not affect the intensity of the GFP fluorescence signals from the progeny (Fig. 5) or the percentage of embryos expressing GFP (45–57% for sur-5∷GFP and 34–38% for rps-5∷GFP). Therefore, CeMed6, CeMed7, and CeMed10/CeNut2 are required for the expression of developmentally regulated genes, but are dispensable for expression of the ubiquitously expressed genes tested in this study.
The gene-specific functions of the C. elegans Mediator homologs were confirmed further by the general requirement of CeTFIIB for the expression of both developmentally regulated and constitutively expressed genes. When CeTFIIB dsRNA was injected into ceh-13∷GFP animals, none of their progeny showed GFP expression (0 of 55 examined) (Fig. 5). Similarly, only 3% of the progenies expressed a strong GFP signal when CeTFIIB dsRNA was injected into rps-5∷GFP animals (3 of 89 examined; Fig. 5). In addition, more than 95% of the embryos obtained from animals injected with CeTFIIB dsRNA died earlier than the lethal stage caused by RNAi of Mediator homologs (data not shown). These results suggest that C. elegans Mediator genes have gene-specific functions, as do their yeast Mediator homologs. The identification of a distinct group of genes regulated by each of the C. elegans Mediator proteins is essential to the understanding of the complex regulatory mechanisms that are operating in developmental stage-specific gene expression.
Acknowledgments
We thank Drs. J. Fleenor, A. Fire, F. Mueller, M. Han, A. Sluder, A. Coulson, and Y. Kohara and the Caenorhabditis Genetics Center (St. Paul, MN) for providing the nematode strains and genomic clones. The OC1D4 mAb against P granules developed by Dr. S. Strome was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City). We also thank Dr. K. LaMarco for critical reading. This work was supported by grants from Samsung Biomedical Research Institute (B-98-18) to Y.-J. K. and the Nondirected Research Fund, Korea Research Foundation, 1998, to Y.-J.K. and J.H.L.
Abbreviations
- TAF
TATA-binding protein (TBP)-associated factor
- pol II
RNA polymerase II
- RNAi
RNA interference
- dsRNA
double-stranded RNA
- dsRNAi
dsRNA interference
- GFP
green fluorescent protein
References
- 1.Burley S K, Roeder R G. Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 2.Roeder R G. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 3.Bjorklund S, Almouzni G, Davidson I, Nightingale K P, Weiss K. Cell. 1999;96:759–767. doi: 10.1016/s0092-8674(00)80586-3. [DOI] [PubMed] [Google Scholar]
- 4.Hampsey M. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travers A. Cell. 1999;96:311–314. doi: 10.1016/s0092-8674(00)80543-7. [DOI] [PubMed] [Google Scholar]
- 6.Workman J L, Kingston R E. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 7.Wolffe A P, Hayes J J. Nucleic Acids Res. 1999;27:711–720. doi: 10.1093/nar/27.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugh B F, Tjian R. J Biol Chem. 1992;267:679–682. [PubMed] [Google Scholar]
- 9.Hoffmann A, Horikoshi M, Wang C K, Schroeder S, Weil P A, Roeder R G. Genes Dev. 1990;4:1141–1148. doi: 10.1101/gad.4.7.1141. [DOI] [PubMed] [Google Scholar]
- 10.Moqtaderi Z, Bai Y, Poon D, Weil P A, Struhl K. Nature (London) 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 11.Walker S S, Reese J C, Apone L M, Green M R. Nature (London) 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Zwicker J, Szymanski P, Levine M, Tjian R. Proc Natl Acad Sci USA. 1998;95:13483–13488. doi: 10.1073/pnas.95.23.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Cote J, Xue Y, Zhou S, Khavari P A, Biggar S R, Muchardt C, Kalpana G V, Goff S P, Yaniv M, et al. EMBO J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon H, Imbalzano A N, Khavari P A, Kingston R E, Green M R. Nature (London) 1994;370:477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- 15.Reyes J C, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. EMBO J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elfring L K, Daniel C, Papoulas O, Deuring R, Sarte M, Moseley S, Beek S J, Waldrip W R, Daubresse G, DePace A, et al. Genetics. 1998;148:251–265. doi: 10.1093/genetics/148.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Mello C. Genes Dev. 1998;12:943–955. doi: 10.1101/gad.12.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleher R J, III, Flanagan P M, Kornberg R D. Cell. 1990;61:1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y C, Kim Y J. Mol Cell Biol. 1998;18:5364–5370. doi: 10.1128/mcb.18.9.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers L C, Gustafsson C M, Hayashibara K C, Brown P O, Kornberg R D. Proc Natl Acad Sci USA. 1999;96:67–72. doi: 10.1073/pnas.96.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu W, Malik S, Ito M, Yuan C X, Fondell J D, Zhang X, Martinez E, Qin J, Roeder R G. Mol Cell. 1999;3:97–108. doi: 10.1016/s1097-2765(00)80178-1. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Zhang Y, Cho H, Rickert P, Lees E, Lane W, Reinberg D. Mol Cell. 1998;2:213–222. doi: 10.1016/s1097-2765(00)80131-8. [DOI] [PubMed] [Google Scholar]
- 24.Boyer T G, Martin M E, Lees E, Ricciardi R P, Berk A J. Nature (London) 1999;399:276–279. doi: 10.1038/20466. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y W, Veschambre P, Erdjument-Bromage H, Tempst P, Conaway J W, Conaway R C, Kornberg R D. Proc Natl Acad Sci USA. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Nature (London) 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 27.Naar A M, Beaurang P A, Zhou S, Abraham S, Solomon W, Tjian R. Nature (London) 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 28.Ryu S, Zhou S, Ladurner A G, Tjian R. Nature (London) 1999;397:446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- 29.Fondell J D, Guermah M, Malik S, Roeder R G. Proc Natl Acad Sci USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 31.Lichtsteiner S, Tjian R. EMBO J. 1995;14:3937–3945. doi: 10.1002/j.1460-2075.1995.tb00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mello C C, Kramer J M, Stinchcomb D, Ambros V. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han S J, Lee Y C, Gim B S, Ryu G H, Park S J, Lane W S, Kim Y-J. Mol Cell Biol. 1999;19:979–988. doi: 10.1128/mcb.19.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y C, Park J M, Min S, Han S J, Kim Y-J. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Nature (London) 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 36.Wittmann C, Bossinger O, Goldstein B, Fleischmann M, Kohler R, Brunschwig K, Tobler H, Muller F. Development. 1997;124:4193–4200. doi: 10.1242/dev.124.21.4193. [DOI] [PubMed] [Google Scholar]
- 37.Sluder A E, Lindblom T, Ruvkun G. Dev Biol. 1997;184:303–319. doi: 10.1006/dbio.1997.8544. [DOI] [PubMed] [Google Scholar]
- 38.Gu T, Orita S, Han M. Mol Cell Biol. 1998;18:4556–4564. doi: 10.1128/mcb.18.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]