Abstract
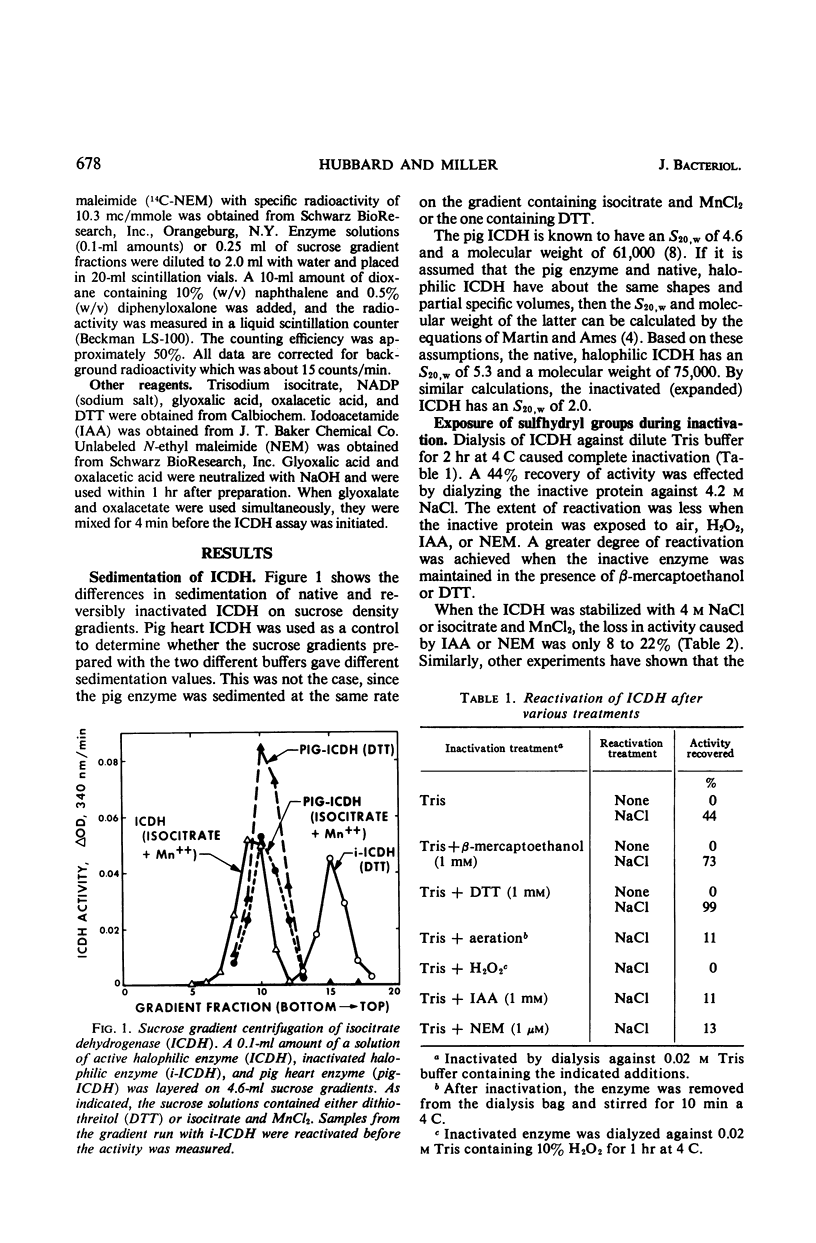
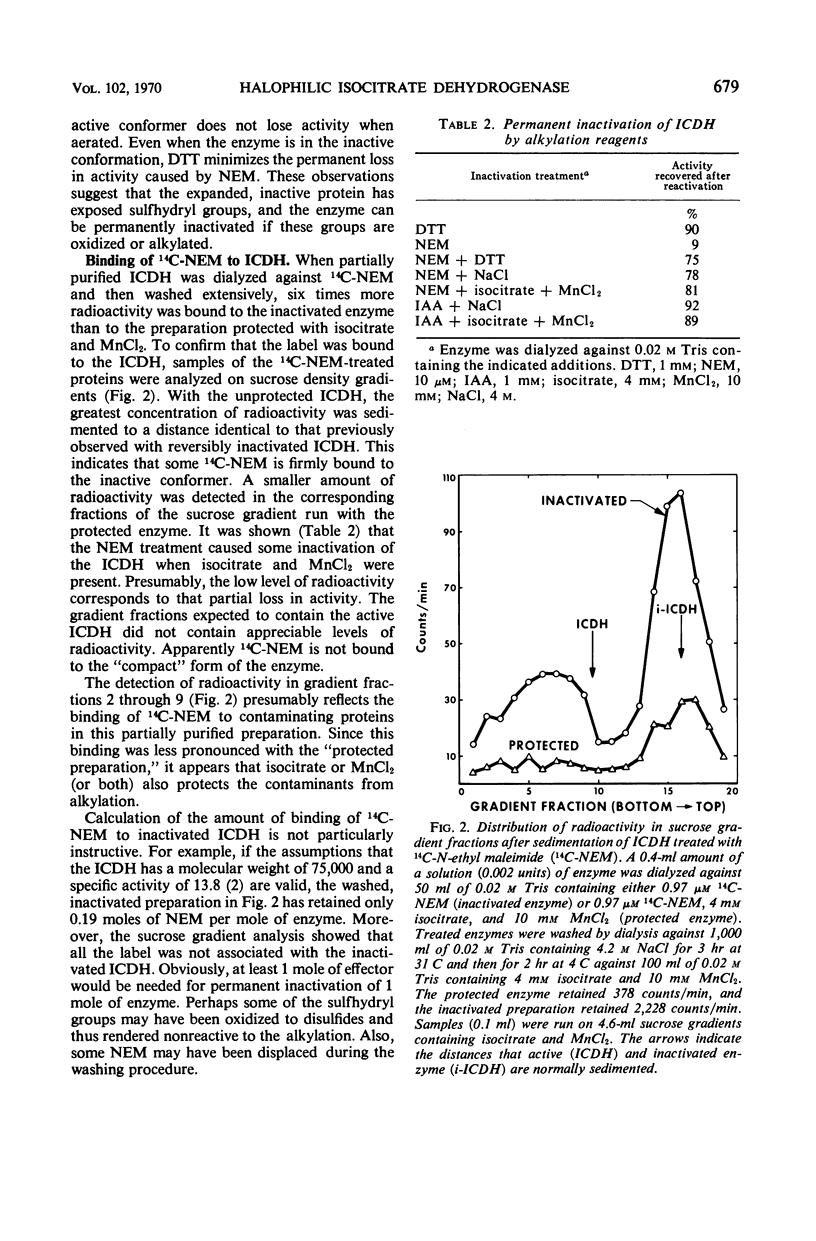
The nicotinamide adenine dinucleotide phosphate-specific isocitrate dehydrogenase (ICDH) of Halobacterium cutirubrum is rapidly inactivated at low NaCl levels. From sucrose gradient analysis, it was estimated that the active ICDH has an S20,w of 5.3 and a molecular weight of 75,000. The inactivation by removal of NaCl causes an unfolding of the protein yielding a less-compact conformer with an S20,w of 2.0. This inactivation apparently causes internal sulfhydryl groups to be exposed. Over 90% of the initial activity can be restored by dialyzing the inactivated ICDH against 4 m NaCl, provided that the exposed sulfhydryl groups are protected with dithiothreitol. The ICDH is permanently inactivated when the sulfhydryl groups are oxidized or alkylated. The alkylation of the inactive ICDH was demonstrated by treatment with 14C-N-ethyl maleimide. Sucrose gradient analysis showed that 14C was bound to a protein with sedimentation properties identical to that of reversibly inactivated ICDH, i.e., an S20,w of 2.0. Much less 14C was bound when active ICDH was treated with 14C-N-ethyl maleimide. The H. cutirubrum ICDH resembles other bacterial isocitrate dehydrogenases in being susceptible to concerted feedback inhibition by oxalacetate and glyoxalate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HOLMES P. K., HALVORSON H. O. PROPERTIES OF A PURIFIED HALOPHILIC MALIC DEHYDROGENASE. J Bacteriol. 1965 Aug;90:316–326. doi: 10.1128/jb.90.2.316-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S., Miller A. B. Purification and reversible inactivation of the isocitrate dehydrogenase from an obligate halophile. J Bacteriol. 1969 Jul;99(1):161–168. doi: 10.1128/jb.99.1.161-168.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- SIEBERT G., DUBUC J., WARNER R. C., PLAUT G. W. The preparation of isocitric dehydrogenase from mammalian heart. J Biol Chem. 1957 Jun;226(2):965–975. [PubMed] [Google Scholar]
- Shiio I., Ozaki H. Concerted inhibition of isocitrate dehydrogenase by glyoxylate plus oxalacetate. J Biochem. 1968 Jul;64(1):45–53. doi: 10.1093/oxfordjournals.jbchem.a128861. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]


