Abstract
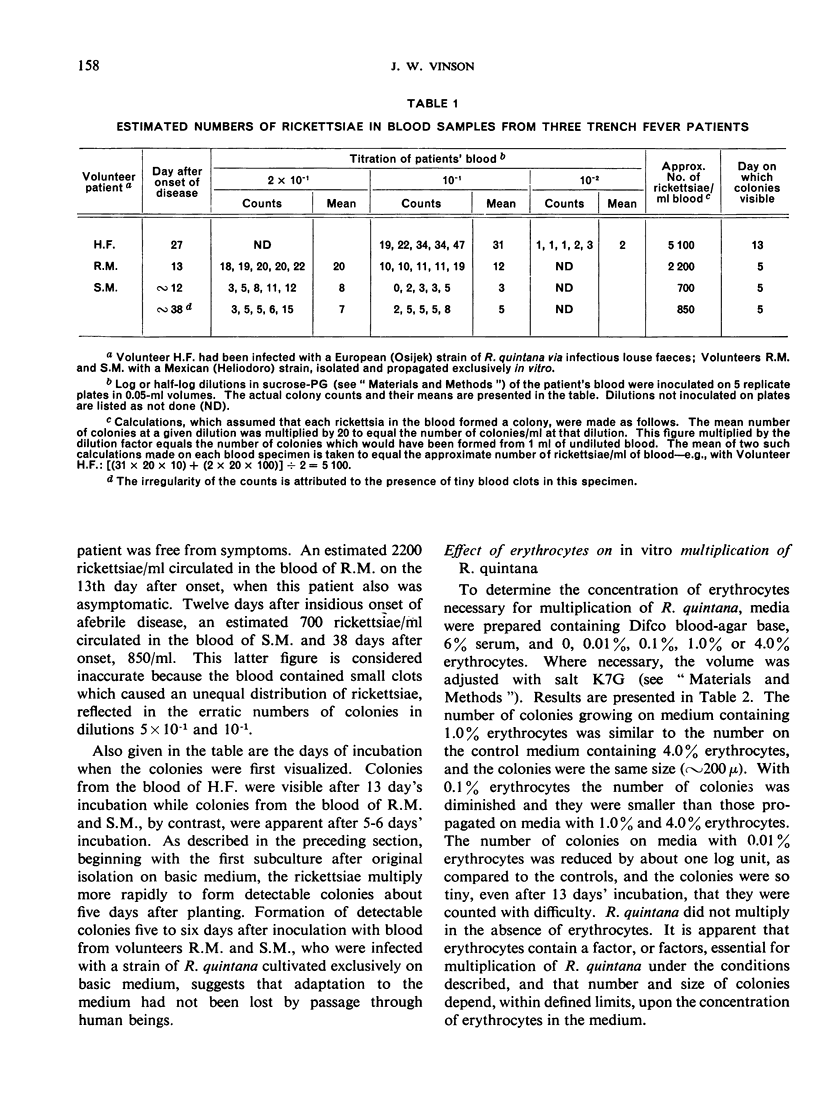
Although trench fever appears to be endemic in many areas of the world, recognition of the disease has been handicapped by the difficulties of making a clinical diagnosis and the unavailability of a simple laboratory procedure to establish the etiology. The author describes a method for the in vitro cultivation of Rickettsia quintana that provides a relatively simple means for the laboratory diagnosis of trench fever. R. quintana can be propagated with ease from the blood of patients directly on blood agar incubated at 37°C for 12-14 days under a gas tension of 5% CO2 in air. The number of rickettsiae circulating in the patient's peripheral blood can be quantitated. The protracted rickettsiaemia in trench fever makes for a relatively long period during which blood culture can be usefully employed.
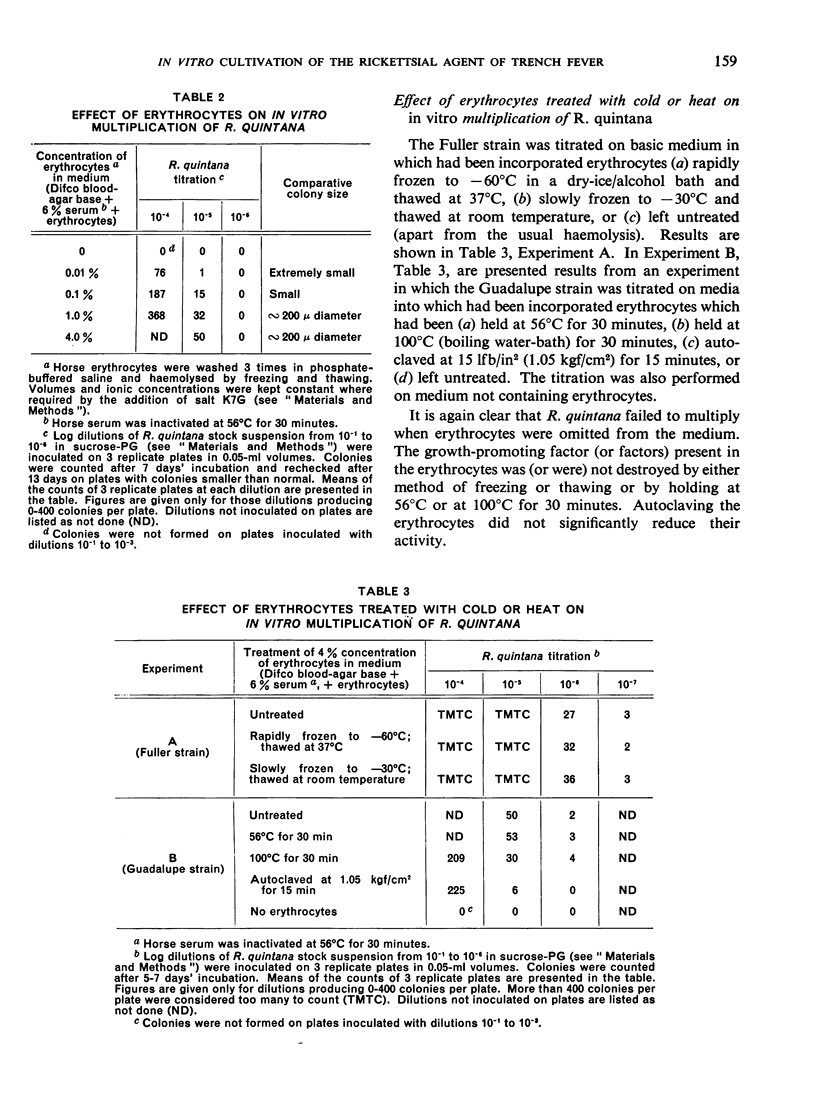
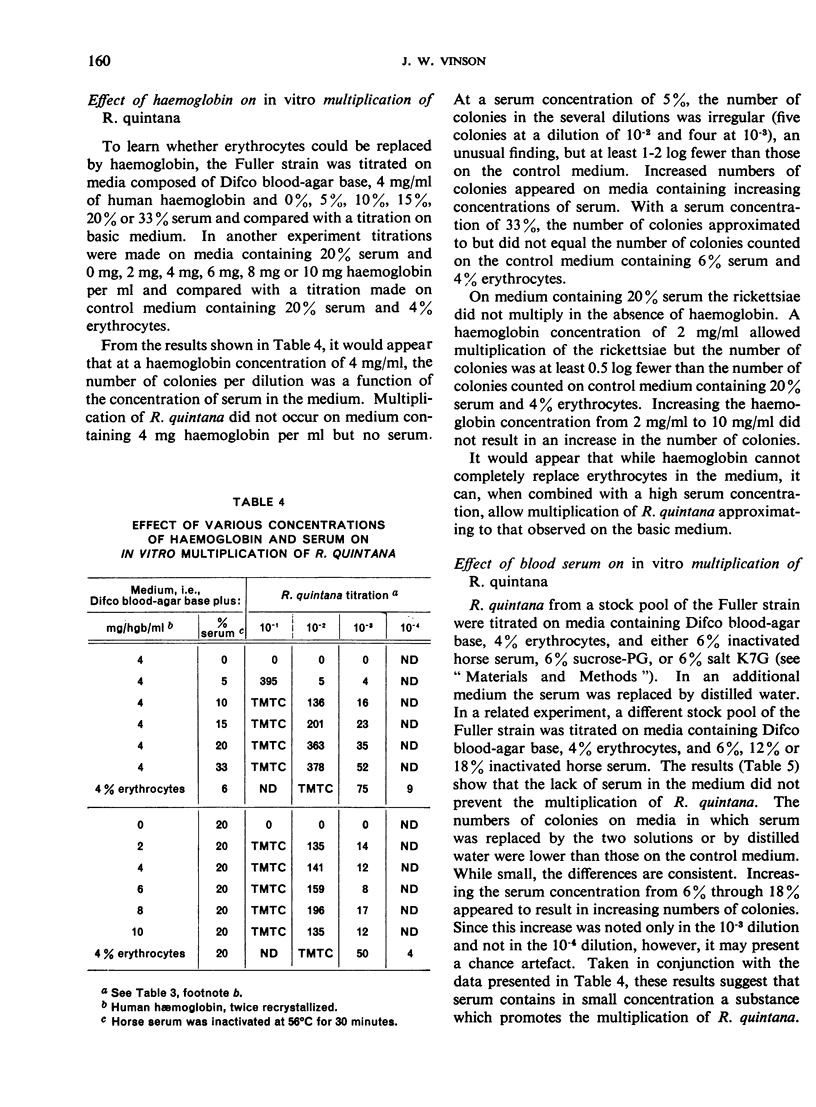
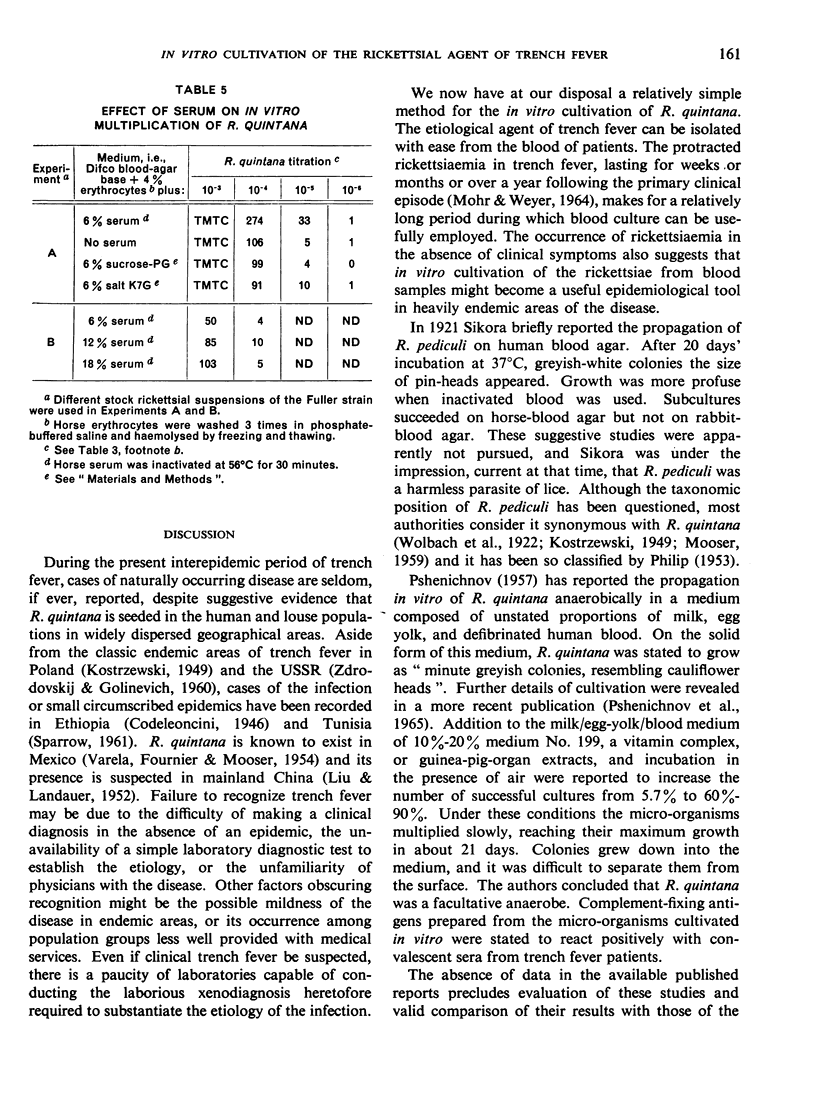
In the course of studies with the method described, it was found that erythrocytes contain a factor (or, possibly, factors) essential for multiplication of R. quintana; this factor is cryostable and thermostable and may be haemoglobin. Blood serum also promotes multiplication.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- HARSHMAN S., JOHNSON J., PRICE W. H. The effect of red blood cells on experimental infection of white rats with the R strain of Rickettsia rickettsii. Am J Hyg. 1958 Jan;67(1):109–117. doi: 10.1093/oxfordjournals.aje.a119913. [DOI] [PubMed] [Google Scholar]
- ITO S., VINSON J. W. FINE STRUCTURE OF RICKETTSIA QUINTANA CULTIVATED IN VITRO AND IN THE LOUSE. J Bacteriol. 1965 Feb;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHR W., WEYER F. SPAETRUECKFAELLE BEI WOLHYNISCHEM FIEBER. Dtsch Med Wochenschr. 1964 Feb 7;89:244–248. doi: 10.1055/s-0028-1111012. [DOI] [PubMed] [Google Scholar]
- Mooser H., Varela G., Pilz H. EXPERIMENTS ON THE CONVERSION OF TYPHUS STRAINS. J Exp Med. 1934 Jan 31;59(2):137–157. doi: 10.1084/jem.59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIP C. B. Nomenclature of the rickettsiaceae pathogenic to vertebrates. Ann N Y Acad Sci. 1953 Mar 31;56(3):484–494. doi: 10.1111/j.1749-6632.1953.tb30240.x. [DOI] [PubMed] [Google Scholar]
- VINSON J. W., FULLER H. S. Studies on trench fever. I. Propagation of Rickettsia-like microorganisms from a patient's blood. Pathol Microbiol (Basel) 1961;24(Suppl):152–166. [PubMed] [Google Scholar]


