Abstract
Primary biliary cirrhosis (PBC) is a liver specific autoimmune disease characterized by antimitochondrial autoantibodies (AMAs) and progressive destruction of intrahepatic bile ducts. The elucidation of early events in the induction of tissue inflammation and autoimmunity in PBC has been hampered by the cryptic onset of the disease, the practical limitations in accessing human liver and the lack of a suitable animal model. Three spontaneous autoimmune biliary disease (ABD) mouse models, including NOD.c3c4 and NOD.c3c4-derived, dominant negative TGF-β receptor II (dnTGFβRII), and IL-2Rα−/− mouse models have been reported recently. All of these spontaneous ABD animal models for PBC develop features characteristic of human PBC, including biliary lymphocytic infiltrates, AMAs directed against the inner lipoyl domain of the E2 subunits of the pyruvate dehydrogenase complex (PDC-E2), and elevated serum levels of IFN-γ and TNF-α. Although some differences exist, these models provide important tools to study the genetic basis and the role of immunoregulation in autoimmune cholangitis and also to allow observation of the earliest stages of loss of tolerance.
Keywords: autoimmune biliary disease; NODc3c4, IL-2α−/−; Dominant negative TGF-β receptor II; xenobiotics
Introduction
Primary biliary cirrhosis (PBC) is a liver specific autoimmune disease characterized by antimitochondrial autoantibodies (AMAs) and progressive destruction of intrahepatic bile ducts. Over the past two decades, the target mitochondrial autoantigens identifying the T and B cell epitopes, the innate and adaptive immune responses, the immunobiology of biliary epithelium, and the mechanisms of tissue destruction in PBC have been studied [1, 2]. While this work has illuminated many of the late stage events, it has been extremely difficult to study initiating events because of the cryptic nature of disease onset which prevents identification of patients in the earliest stages. In other autoimmune diseases, understanding the immune process has been markedly enhanced by the availability of informative animal models, such as the NZB × NZW F1 and MRL/lpr mouse for systemic lupus erythematosus (SLE) and NOD models of type 1 diabetes (T1D). Until recently there has been no such model in PBC. Herein, we will review three spontaneous mouse models and two induced models of PBC._
Clinical features and the immune profile of patients with PBC
PBC is an autoimmune liver disease which predominantly affects middle-aged women, characterized by the presence of AMAs, infiltration of lymphocytes in portal tracts and destruction of intrahepatic small bile ducts, causing liver fibrosis and eventually liver failure [1–3]. Lymphocytic infiltration and granuloma formation are often seen in the hepatic lesions of PBC. The presence of AMAs is the hallmark of PBC and can be detected years before disease onset [4]. The autoantigens recognized by AMAs are located on the inner mitochondrial membrane and identified as members of the 2-oxo-acid dehydrogenase enzyme complexes (2-OADC) which include the E2 subunit of the pyruvate dehydrogenase complex (PDC), the branched chain 2-oxo-acid dehydrogenase complex (BCOADC), the α-ketoglutaric acid dehydrogenase complex (OGDC), and the dihydrolipoamide dehydrogenase binding protein [5]. Among them, the major autoantigen in PBC is the E2 subunit of PDC (PDC-E2) [6]. The major epitope of PDC-E2 has been mapped to its inner lipoyl domain [7]. Interestingly, the dominant autoreactive B cell epitope (peptides 167–186) and the T cell epitopes (CD4+ T cell: peptides 163–176; CD8+ T cell: peptides 159–167) from patients with PBC all appear to localize to the inner lipoyl domain of the PDC-E2 [7–10].
The presence of T lymphocytes infiltrating portal tracts and the detectable expression of class II MHC on biliary epithelium [11] suggests that liver-infiltrating autoreactive T cells mediate the destruction of biliary epithelial cells. Autoreactive CD4+ and CD8+ T cells against PDC-E2 in the periphery are accompanied by a disease specific 100–150 fold increase in the precursor frequency of PDC-E2 specific CD4+ T cells in the hilar lymph nodes and liver from the same PBC patients [7], and a 10–15 fold enrichment of PDC-E2 specific CD8+ T cells in liver compared to PBMC [9]. In addition, CD4+ T cells are more frequently found in inflamed portal tracts compared to CD8+ T cells; however, CD8+ T cells are more dominant in areas of bile duct destruction [12], which is suggestive of their active participation in tissue damage. Infiltrating T cells appear to be polyclonal, and skewed towards a Th1 profile with high IFN-γ production and low levels of IL-4 [13].
Defects of regulatory T cells (Tregs) may also accelerate T cell autoimmunity in PBC [14]. PBC patients have been found to have fewer peripheral CD4+CD25+ Tregs than healthy controls. Moreover, the number of Foxp3-expressing Tregs in portal tracts affected by PBC is markedly lower compared with other liver diseases including chronic hepatitis C and autoimmune hepatitis. Interestingly, healthy daughters and sisters of patients with PBC also exhibit a reduced peripheral Treg frequency, implicating genetic factors in the relative Treg deficiency observed in PBC. In addition, the presence of AMAs and a PBC-like liver disease has been observed in a child with an IL-2Rα (CD25) homozygous deficiency [15]. These findings suggest that Treg deficiency may play a role in the loss of tolerance in PBC.
Patients with PBC also manifest elevated sera levels of IgM [16]. This elevation of sera IgM is associated with an increased response to CpGB, but not CpGA, in patients with PBC, with a corresponding upregulation of TLR9. This increased responsiveness is found primarily in CD27+ memory B cells and requires the presence of T cells as well as plasmacytoid dendritic cells. Interestingly, this elevation of sera IgM precedes the onset of disease and is also found in AMA negative PBC [17]. In addition, CpGB-stimulated B cells from patients with PBC produce AMA and express TLR9, CD86 and one of the potassium channels, KCa3.1 [18].
There is a female predominance in PBC and it is 50–100 fold higher frequency in first degree relatives having a member with PBC than the general population [19]. There is no particular HLA haplotype associated with the occurrence of PBC, even in familial cases. The involvement of an abnormal X chromosome in the female susceptibility to PBC has been suggested [20] and the frequency of X monosomy in peripheral blood cells from female PBC patients is significantly higher than in chronic hepatitis C and healthy controls using fluorescence in situ hybridization. In addition, the concordance rate of PBC in identical twins is approximately 60%, the highest of any autoimmune disease other than celiac sprue [21]. The importance of a genetic background for the development of PBC is further demonstrated by studying first degree relatives of PBC. As above mentioned, healthy daughters and sisters of PBC patients also exhibited a reduced peripheral Treg frequency. Furthermore, increased plasma levels of interferon-γ-inducible protein 10 (IP-10) and monokine induced by gamma interferon (MIG) were observed not only in patients with PBC but also their healthy daughters and sisters compared to unrelated healthy controls [22].
The early histopathology of PBC involves damage to biliary epithelium, presumably mediated by lymphocytes that surround and often infiltrate the duct [23]. Epithelial cells may be vacuolated, shrunken, and pyknotic. Necrotic bile ducts are often located at the center of large granuloma-like lesions that consist of histiocytes, lymphocytes, plasma cells, eosinophils, and occasionally true giant cells [24]. At this stage, inflammation remains confined to the portal triads. The lymphocytes that infiltrate the ductular epithelial cells directly and presumably destroy them are CD8 cells [9]. The majority of lymphocytes in the portal triads are CD4 cells (helper–inducer cells) [25] although B cells are also seen. As the disease progresses to stage II, many portal triads become scarred, inflammatory cells spill out of the triads into the surrounding periportal parenchyma, normal bile ducts cut in cross section disappear including atypical, poorly formed, tortuous bile ducts with no obvious lumens [26]. Periportal hepatocytes become vacuolated and surrounded by foamy macrophages, a process termed biliary piecemeal necrosis [27]. In stage III, scarring progresses to the point at which fibrous septa link many adjoining portal triads. Stage IV is frank cirrhosis [28]
NOD.c3c4 and NOD.c3c4-derived mouse lines
NOD.c3c4
The NOD mouse is a well-characterized spontaneous model of autoimmune type 1 diabetes (T1D) with immune-mediated pancreatic islet destruction. NOD mice exhibit multiple immune similarities with patients with T1D, including the presence of pancreas-specific autoantibodies and autoreactive CD4+ and CD8+ T cells, functional abnormalities of T cells, NK cells and perhaps B cells, deficiencies in NKT cells and CD4+CD25+ Tregs [29–31]. In addition to autoimmune diabetes, NOD and NOD-related strains are prone to develop other autoimmune syndromes, including autoimmune sialitis [32], autoimmune thyroiditis [33], autoimmune peripheral polyneuropathy [34], and Sjogren’s-like syndrome [35]. Thus, similar to some humans with T1D, the NOD mouse combines an overall genetic propensity for multi-organ autoimmunity with specific targets not limited to organs of the endocrine system.
The NOD.c3c4 mouse, the first spontaneous mouse model of ABD, was discovered unexpectedly. It was originally constructed to demonstrate that complete protection from diabetes ensued when B6/B10 insulin dependent diabetes (Idd) resistance loci (from chromosomes 3 and 4) were placed on the NOD background [36]. However, while resistant to diabetes, it develops biliary lymphocytic infiltrates, autoantibodies, and progressive, often fatal, biliary obstruction [37]. Moreover, the disease can be transferred by splenocytes to naïve mice, proving an autoimmune etiology [37].
The NOD.c3c4 mouse and patients with PBC share several comparable clinical and autoimmune phenotypes. Histopathological examination demonstrates that NOD.c3c4 mice develop extensive peri-biliary lymphocytic infiltrates, granuloma-like lesions, and chronic nonsuppurative destructive cholangitis in the liver [38]. Furthermore, 50–60% of the NOD.c3c4 mice produce AMAs that react with the inner lipoyl domain of PDC-E2 and inhibit PDC enzyme function. Immunohistochemistry clearly demonstrates that CD3+ cells infiltrate areas of active cyst formation. Moreover, the CD3+ cells are found directly infiltrating biliary epithelial cells. In addition, administration of CD3+ T cell depleting anti-CD3 antibodies in young NOD.c3c4 mice significantly reduces disease onset in these mice [38]. These results suggest an active role for T cells in the PBC disease process.
However, distinct from human PBC, in NOD.c3c4 mice, common bile duct dilation is observed as early as 3 weeks of age and up to 90% of mice develop progressive intra-hepatic biliary cyst formation accompanied by adjacent lymphocytic infiltration by the age of 30 weeks [37, 38]. A follow-up study shows that Fas expression on cholangiocytes in NOD.c3c4 mice is significantly reduced compared to control mice. In addition, cholangiocytes in NOD.c3c4 mice are proliferative cellular nuclear antigen (PCNA) positive but TUNEL negative [39]. These results suggest that the abnormal cell proliferation and absence of apoptosis contribute to biliary cyst formation in NOD.c3c4 mice and this defect accelerates an autoimmune response in mice genetically susceptible to loss of tolerance.
NOD.c3c4-derived mice: a congenic mapping approach
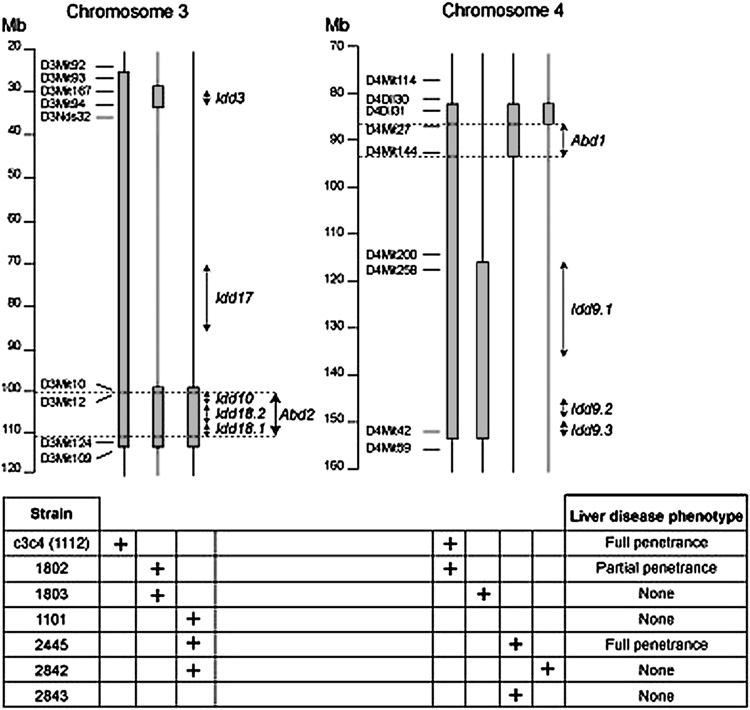
Multiple loci control genetic susceptibility to diabetes in NOD mice. The strongest genetic contribution is the MHC class II molecule, I-Ag7; this allele is necessary but not sufficient for disease [29, 30]. In addition to the MHC locus, other loci contribute to disease development and are termed insulin dependent diabetes (Idd) loci. At present at least 20 potential Idd loci have been identified using congenic mice [40]. This congenic strategy has been applied to define the underlying immunogenetic basis of the Autoimmune biliary disease (ABD) phenotype. The construction of Idd congenic mice demonstrates that several genes are critical for causing liver diseases in NOD.c3c4 mice [37]. For example, neither the NOD.c3 nor the NOD.c4 substrains develop ABD. However, NOD.c3 mice develop liver lymphocytic infiltrates without autoantibody production, while NOD.c4 mice develop antinuclear antibodies (ANAs) but neither AMAs nor liver pathology. When combined in the NOD.c3c4 mouse, these genetic intervals interact synergistically to produce both ABD and AMAs [37]. The critical genetic regions causing liver disease in ABD mice will eventually be identified using the “congenic dissection” strategy, which has involved producing progressively smaller intervals from the critical regions on chromosomes 3 and 4 by selecting recombination events during a backcross to the NOD strain (Figure 1).
Figure 1.
Genetic dissection of ABD in NOD.c3c4-related mice. The upper portion of the figure shows the B6/B10 regions introgressed onto the NOD background in seven congenic strains. The bottom portion of the figure indicates which regions are present in which strains (as marked by a “+”), and whether the strains develop ABD. Two genetic regions proven to be necessary for disease are marked by Abd1 and Abd2.
Dominant negative TGF-β receptor II (dnTGFβRII) mouse
TGF-β is a well established immune regulatory cytokine with pleiotropic effects on cell proliferation, differentiation, migration, and survival that affect multiple biological processes, including carcinogenesis, fibrosis, wound healing, and immune responses. Importantly, it exerts suppressive effects on the survival, activation, proliferation, and effector functions of several key immune players such as T cells, B cells, natural killer cells, macrophages and dendritic cells [41, 42]. The generation of TGF-β1−/− mice demonstrated a critical role for this cytokine in inhibiting inflammation and autoimmune diseases [43]. TGF-β produced by Th3 cells in mucosal tissues, such as the gut, also plays a critical role in mucosal tolerance [44]. In addition, TGF-β has been demonstrated to promote the development of peripheral FoxP3+ regulatory T cells [45, 46]. The association with a decrease in peripheral Tregs in PBC patients [14], the description of PBC as a mucosal disease [47], and the role of TGF-β in immune modulation, make TGFβ deficient mouse an interesting model for PBC. However, generic TGF-β−/− mice have a life span of 3–4 weeks [43] and are not ideal for study of chronic disease.
In contrast, dnTGFβRII mice have an over-expression of a dominant-negative form of TGFβ receptor type II under the control of the CD4 promoter and spontaneously develops features characteristic of PBC [48]. These features include spontaneous production of AMAs reacting to the mitochondrial autoantigens, PDC-E2, BCOADC-E2 and OGDC-E2, and these AMAs inhibit PDC-E2 activity and reactivity with the inner lipoyl domain of the respective E2 subunits. Moreover, lymphocytic infiltration in the liver with periportal inflammation is similar to the histological profile in human PBC. Serum levels of cytokines such as TNFα, IFNγ, IL-12p40 and IL-6 in dnTGFβRII mice are higher than control mice. In the liver, both the frequency and the total number of CD3+ cells among the total lymphoid cell infiltrate are significantly increased in dnTGFβRII mice. In addition, the absolute number of hepatic invariant NKT (iNKT) cells is significant increased compared to control mice.
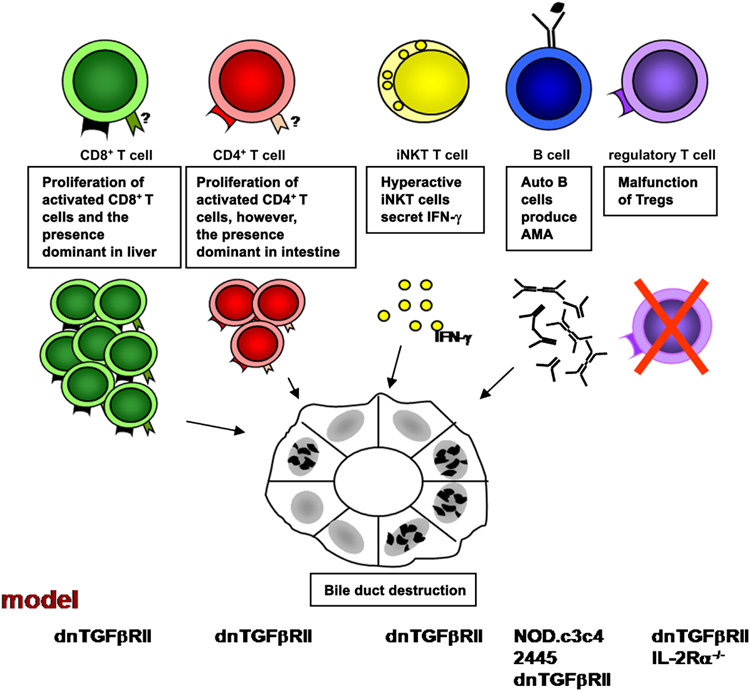
While the total number of intrahepatic CD4+ lymphocytes is increased, the percentage of CD4+ cells within the CD3+ population does not increase. In contrast, the CD8+ population is significantly amplified in total number as well as percentage in the CD3+ population compared with controls. These features result in a change in the ratio of CD4+ to CD8+ T cells biased toward the CD8+ population [48]. This finding is particularly interesting, considering previous reports of an increase in precursors of CTLs in the blood of patients in the early stages of PBC versus advanced stages and the 10-fold increase of autoantigen-specific CTLs within the liver as compared with the circulating pool [9]. The biased ratio of CD4 to CD8 in the liver of dnTGFβRII mice is due to the dominant migration of CD8+ T cells. Rag-1−/− mice adoptively transferred with CD8+ T cells from dnTGFβRII mice demonstrate a significant expansion of T cells and the presence of portal tract infiltrates. In contrast, Rag-1−/− mice adoptively transferred with CD4+ T cells from dnTGFβRII mice also demonstrate an increased number of T cells but they do not home or focus within the portal tracts. The different homing pattern may explain the increased number of CD8+ T cells in liver of PBC patients (Figure 2).
Figure 2.
Immune cells are involved in the early pathogenesis of PBC. T cells mediating the destruction of bile duct in PBC is documented in the dnTGFβRII model by adoptive transfer study. Rag-1−/− mice transferred with CD8+ T cells from dnTGFβRII mice demonstrate a significant expansion of T cells and the presence of portal tract infiltrate. However, the specific homing receptors for CD4+ and CD8+ T cells have not been identified. iNKT cells promote the development of PBC in early stages by secreting IFN-γ. Anti-PDC-E2 autoantibodes are detected in NOD.c3c4, 2445, dnTGFβRII, and IL-2Rα−/− mouse models. The presence of liver disease similar to human PBC in dnTGFβRII and IL-2Rα−/− mouse models indicates the importance of Tregs in PBC.
While CD1d expression and the frequency of invariant NKT (iNKT) cells are both increased in PBC liver [49, 50] the percentage as well as the absolute numbers of hepatic iNKT cells are augmented in this dnTGFβRII mouse model [48]. Furthermore, CD1d−/−dnTGFβRII mice exhibit significantly decreased hepatic lymphoid cell infiltrates and milder cholangitis compared to CD1d+/−dnTGFβRII mice. In addition, there is a significant increase in the production of IFN-γ in hepatic iNKT cells activated by α-galactosylceramide (α-GalCer) in young but not older dnTGFβRII mice. These results suggest iNKT cells could promote the development of PBC in early stages by secreting IFN-γ (Chuang, et al., Hepatology, in press; Figure 2).
Interleukin-2 receptor α (IL-2Rα, CD25) −/− mice
IL-2 is critical for the development and peripheral expansion of CD4+CD25+ Tregs, which promote self-tolerance by suppressing T cell responses in vivo [51]. IL-2Rα−/− mice develop severe anemia and lymphoproliferative autoimmune disorders as well as inflammatory bowel disease, and between 8 and 20 weeks, 25–50% of IL-2Rα−/− mice die from severe hemolytic anemia [52].
IL-2Rα−/− mice develop portal inflammation and biliary ductular damage similar to human PBC [53]. CD4+ and CD8+ T cells predominate among portal cell infiltrates and significantly increased numbers of CD8+ cells are observed. Serum levels of cytokines such as TNFα, IFNγ, IL-12p40 and IL-6 in IL-2Rα−/− mice are higher than that of control mice. Importantly, IL-2Rα−/− mice produce AMAs against the inner lipoyl domain of PDC-E2 [53] similar to PBC patients and the other spontaneous ABD mouse models. What about the human model for IL-2 deficiency?
Induced models
The autoimmune liver disease PBC is characterized by the breakdown of self tolerance to self antigens such as PDC-E2. Administration of PDC-E2 with adjuvant to mice produces AMA but not antigen specific bile duct disease [54]. However, portal tract inflammatory infiltrates are seen in the covalent modified (biotinylated) PDC-sensitized mice [55]. Another approach of breakdown tolerance is xenobiotic immunization. Guinea pigs immunized with xenobiotics, such as 6-bromohexanoate, conjugated with a BSA not only develop AMA similar to human PBC, but also develop PBC-like liver lesions including significant lymphoid cell infiltrates surrounding damaged bile ducts, variable swollen lymphoplasmacytes surrounding interlobular bile duct and bile duct loss [56, 57] unlike the animal models the biliary tract disease is confined to the interlobular bile ducts as in PBC. These models suggest that tolerance breakdown by either modification of the self antigens or molecular mimicry leads to the pathogenesis of PBC. These models also offer the potential to study several key mechanistic issues which relate to generic induction of autoimmunity, including the role of toll-like receptors, innate immunity, repertoire-dependent immunopathology, autoreactive B cells, T regs, naturally occurring autoantibodies, apoptosis, and the immunological homonculus [58–69].
Conclusion
The features of human and those of the mouse models are compared in Table 1. All of these spontaneous ABD animal models of PBC develop features similar to human PBC, i.e. lymphocytic infiltrates, decreased hepatic CD4/CD8 ratios with age, AMAs against inner lipoyl domain of PDC-E2, and elevated serum levels of IFN-γ and TNF-α. It is noted that no experimental model can provide a true facsimile of the counterpart in the genetically complex outbred human. Immunopathology develops equally in male and female mice of these animal models, compared to the female predominance in human PBC. NOD.c3c4 mice develop increased serum IgA and IgM, dnTGFβRII mouse develops increased serum IgA, IgM and IgG, and IL-2Rα−/− mouse develops increased serum IgA and IgG. However, elevated levels of total IgM in serum are noted in human PBC [17]. PBC is characterized histologically by the chronic destruction of small intrahepatic bile ducts with surrounding portal lymphocytic infiltration [2], features also seen in the liver of all of these mouse models which however develop disease in both the extrahepatic as well as the intrahepatic ducts. However, these mouse models develop additional features that are not found in human PBC. For example, NOD.c3c4 mice develop marked biliary cyst formation [37, 38]. Clusters of macrophages and lymphocytes located in parenchyma and adjacent to the blood vessels are observed in the liver of dnTGFβRII mice [70]. IL-2Rα−/− mice develop an ulcerative colitis-like disease. Taken together, these models serve as important tools to study the genetics and immunoregulation of PBC in the earliest stage of disease (Figure 1 and Figure 2).
Table 1.
Human PBC vs. ABD mouse models: Clinical, histological and immunological observations.
| Human PBC | NOD.c3c4 | 2445 | dnTGFβRII | IL-2Rα−/− | |
|---|---|---|---|---|---|
| General feature | |||||
| Genetic factor | 60% concordance in identical twin | 100% | 100% | 100% | 100% |
| Environment factor | Yes | ? | ? | ? | ? |
| Affected gender | mostly female | both sexes | both sexes | both sexes | both sexes |
| Age of onset | 40–60 ys | 9–10 wks | 9–10 wks | 5–6 wks | 5–6 wks |
| Liver histology | |||||
| specific destruction of small bile ducts | Yes | Yes | Yes | Yes | Yes |
| periductual T cell infiltration | Yes | Yes | Yes | Yes | Yes |
| Granuloma formation | Yes | Yes | Yes | No | No |
| Eosinophilia | Yes | Yes | Yes | No | No |
| Fibrosis | Yes | No | No | No | No |
| Pathological role of lymphocytes | ? | Yes* | ? | Yes* | ? |
| T cell immunity | |||||
| liver infiltration of T cells | Yes | Yes | Yes | Yes | Yes |
| autoreactive CD4+ T cell response | Yes | ? | Yes | Yes | ? |
| target of autoreactive CD4+ T cells | PDC-E2 | ? | PDC-E2 | PDC-E2 | ? |
| reduced Treg frequency | Yes | ? | ? | ? | Yes |
| increased iNKT frequency | Yes | ? | ? | Yes | No |
| B cell immunity | |||||
| Immunoglobulins | IgM, IgG | IgM, IgA | ? | IgM, IgG, IgA | IgA, IgG |
| AMA | Yes | Yes | Yes | Yes | Yes |
| Dominant AMA target protein | PDC-E2 | PDC-E2 | PDC-E2 | PDC-E2 | PDC-E2 |
| Dominant Epitope | Lipoyl domain | Lipoyl domain | Lipoyl domain | Lipoyl domain | Lipoyl domain |
| ANA | Yes | Yes | No | ? | Yes |
| Serum biochemistry | |||||
| increased alkaline phosphatase | Yes | ? | ? | ? | ? |
| serum cytokines | |||||
| increased IFN-γ | Yes | Yes | ? | Yes | Yes |
| increased TNF-α | Yes | Yes | ? | Yes | Yes |
adoptive transfer of splenocytes causes disease.
Acknowledgments
Financial Support: National Institutes of Health grant DK39588.
Abbreviations
- ABD
autoimmune biliary disease
- AMA
antimitochondrial autoantibodies
- ANA
antinuclear antibodies
- dnTGFβRII
dominant negative TGF-β receptor II
- Idd
insulin dependent diabetes
- iNKT
invariant natural killer T
- PBC
primary biliary cirrhosis
- PDC-E2
the E2 subunits of the pyruvate dehydrogenase complex
- Treg
regulatory T cell
- T1D
type 1 diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, van de Water J, LaRusso NF, Gershwin ME, Gores GJ, Odin JA. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He XS, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell Immunol. 2006;239:1–13. doi: 10.1016/j.cellimm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 4.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Gershwin ME. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 5.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 6.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe KB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kita H, Ansari AA, He XS, Lian ZX, Van de Water J, Coppel RL, Luketic V, Kaplan M, Inamori H, Isoda N, Sugano K, Imawari M, Gershwin ME. Proteasome is required for class I-restricted presentation by Fcgamma receptor-mediated endocytosis in primary biliary cirrhosis. J Autoimmun. 2003;21:175–182. doi: 10.1016/s0896-8411(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 9.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanimoto H, Shimoda S, Nakamura M, Ishibashi H, Kawano A, Kamihira T, Matsushita S, Gershwin ME, Harada M. Promiscuous T cells selected by Escherichia coli: OGDC-E2 in primary biliary cirrhosis. J Autoimmun. 2003;20:255–263. doi: 10.1016/s0896-8411(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 11.Leon MP, Bassendine MF, Wilson JL, Ali S, Thick M, Kirby JA. Immunogenicity biliary epithelium: investigation of antigen presentation to CD4+ T cells. Hepatology. 1996;24:561–567. doi: 10.1002/hep.510240317. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto E, Lindor KD, Homburger HA, Dickson ER, Czaja AJ, Wiesner RH, Ludwig J. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049–1055. doi: 10.1016/s0025-6196(12)60897-0. [DOI] [PubMed] [Google Scholar]
- 13.Harada K, Van de Water J, Leung PS, Coppel RL, Ansari A, Nakanuma Y, Gershwin ME. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791–796. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- 14.Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S, Tanaka A, Bowlus CL, Takano Y, Ansari AA, Coppel RL, Gershwin ME. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- 15.Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun. 2006;27:50–53. doi: 10.1016/j.jaut.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Takii Y, Ito M, Komori A, Yokoyama T, Shimizu-Yoshida Y, Koyabu M, Matsuyama M, Mori T, Kamihira T, Daikoku M, Migita K, Yatsuhashi H, Nozaki N, Shimoda S, Ishibashi H. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis. J Autoimmun. 2006;26:138–145. doi: 10.1016/j.jaut.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, Coppel RL, Gershwin ME. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Moritoki Y, Lian ZX, Wulff H, Yang GX, Chuang YH, Lan RY, Ueno Y, Ansari AA, Coppel RL, Mackay IR, Gershwin ME. AMA production in primary biliary cirrhosis is promoted by the TLR9 ligand CpG and suppressed by potassium channel blockers. Hepatology. 2007;45:314–322. doi: 10.1002/hep.21522. [DOI] [PubMed] [Google Scholar]
- 19.Selmi C, Invernizzi P, Keeffe KB, Coppel RL, Podda M, Rossaro L, Ansari AA, Gershwin ME. Epidemiology and pathogenesis of primary biliary cirrhosis. J Clin Gastroenterol. 2004;38:264–271. doi: 10.1097/00004836-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 21.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M, Gershwin ME. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, Chiang BL, Ansari AA, Tsuneyama K, Coppel RL, Gershwin ME. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126–132. doi: 10.1016/j.jaut.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Salunga TL, Cui ZG, Shimoda S, Zheng HC, Nomoto K, Kondo T, Takano Y, Selmi C, Alpini G, Gershwin ME, Tsuneyama K. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J Autoimmun. 2007;29:78–86. doi: 10.1016/j.jaut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi H, Matsumura T, Kumakiri M, Ohkawara A. [Generalized granuloma annulare with primary biliary cirrhosis] Nippon Hifuka Gakkai Zasshi. 1990;100:871–878. [PubMed] [Google Scholar]
- 25.Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, Liu S, Roche TE, Krams SM, Munoz S, Gershwin ME. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723–733. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuer PJ. Ludwig Symposium on biliary disorders--part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc. 1998;73:179–183. doi: 10.4065/73.2.179. [DOI] [PubMed] [Google Scholar]
- 27.Nakanuma Y, Saito K, Unoura M. Semiquantitative assessment of cholestasis and lymphocytic piecemeal necrosis in primary biliary cirrhosis: a histologic and immunohistochemical study. J Clin Gastroenterol. 1990;12:357–362. doi: 10.1097/00004836-199006000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig J. New concepts in biliary cirrhosis. Semin Liver Dis. 1987;7:293–301. doi: 10.1055/s-2008-1040584. [DOI] [PubMed] [Google Scholar]
- 29.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 30.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmun Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa N, Minamimura K, Kodaka T, Maki T. Short administration of polyclonal anti-T cell antibody (ALS) in NOD mice with extensive insulitis prevents subsequent development of autoimmune diabetes. J Autoimmun. 2006;26:225–231. doi: 10.1016/j.jaut.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263:E607–E614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 33.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 34.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjogren's syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26:90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Lyons PA, Hancock WW, Denny P, Lord CJ, Hill NJ, Armitage N, Siegmund T, Todd JA, Phillips MS, Hess JF, Chen SL, Fischer PA, Peterson LB, Wicker LS. The NOD Idd9 genetic interval influences the pathogenicity of insulitis and contains molecular variants of Cd30, Tnfr2, and Cd137. Immunity. 2000;13:107–115. doi: 10.1016/s1074-7613(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 37.Koarada S, Wu Y, Fertig N, Sass DA, Nalesnik M, Todd JA, Lyons PA, Fenyk-Melody J, Rainbow DB, Wicker LS, Peterson LB, Ridgway WM. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 38.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, Leung PS, Cheng C, Mackay IR, Gershwin ME, Ridgway WM. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagome Y, Ueno Y, Kogure T, Fukushima K, Moritoki Y, Ridgway WM, Eric Gershwin M, Shimosegawa T. Autoimmune cholangitis in NOD.c3c4 mice is associated with cholangiocyte-specific Fas antigen deficiency. J Autoimmun. 2007;29:20–29. doi: 10.1016/j.jaut.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Todd JA, Wicker LS. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15:387–395. doi: 10.1016/s1074-7613(01)00202-3. [DOI] [PubMed] [Google Scholar]
- 41.Holzer U, Rieck M, Buckner JH. Lineage and signal strength determine the inhibitory effect of transforming growth factor beta1 (TGF-beta1) on human antigen-specific Th1 and Th2 memory cells. J Autoimmun. 2006;26:241–251. doi: 10.1016/j.jaut.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng J, Dicker B, Du W, Tang F, Nguyen P, Geiger T, Wong FS, Wen L. Converting antigen-specific diabetogenic CD4 and CD8 T cells to TGF-beta producing non-pathogenic regulatory cells following FoxP3 transduction. J Autoimmun. 2007;28:188–200. doi: 10.1016/j.jaut.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, Branch AD, Coppel RL, Ansari AA, Gershwin ME. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32:910–915. doi: 10.1053/jhep.2000.19254. [DOI] [PubMed] [Google Scholar]
- 48.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO, Flavell RA, Kronenberg M, Mackay IR, Gershwin ME. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 49.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL, Kaplan M, Gershwin ME. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 50.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28:620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 51.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 52.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 53.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA, Coppel RL, Mackay IR, Gershwin ME. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 54.Jones DE, Palmer JM, Yeaman SJ, Kirby JA, Bassendine MF. Breakdown of tolerance to pyruvate dehydrogenase complex in experimental autoimmune cholangitis: a mouse model of primary biliary cirrhosis. Hepatology. 1999;30:65–70. doi: 10.1002/hep.510300123. [DOI] [PubMed] [Google Scholar]
- 55.Palmer JM, Robe AJ, Burt AD, Kirby JA, Jones DE. Covalent modification as a mechanism for the breakdown of immune tolerance to pyruvate dehydrogenase complex in the mouse. Hepatology. 2004;39:1583–1592. doi: 10.1002/hep.20248. [DOI] [PubMed] [Google Scholar]
- 56.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, Ansari AA, Coppel RL, Lam KS, Gershwin ME. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–5332. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 57.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J. Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avrameas S, Ternynck T, Tsonis IA, Lymberi P. Naturally occurring B-cell autoreactivity: A critical overview. J Autoimmun. 2007;29:213–218. doi: 10.1016/j.jaut.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007;29:246–249. doi: 10.1016/j.jaut.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Lan RY, Mackay IR, Eric Gershwin M. Regulatory T cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J Autoimmun. 2007;29:272–280. doi: 10.1016/j.jaut.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lang KS, Burow A, Kurrer M, Lang PA, Recher M. The role of the innate immune response in autoimmune disease. J Autoimmun. 2007;29:206–212. doi: 10.1016/j.jaut.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Lutz HU. Homeostatic roles of naturally occurring antibodies: An overview. J Autoimmun. 2007;29:287–294. doi: 10.1016/j.jaut.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Milner J, Ward J, Keane-Myers A, Min B, Paul WE. Repertoire-dependent immunopathology. J Autoimmun. 2007;29:257–261. doi: 10.1016/j.jaut.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papadimitraki ED, Bertsias GK, Boumpas DT. Toll like receptors and autoimmunity: A critical appraisal. J Autoimmun. 2007;29:310–318. doi: 10.1016/j.jaut.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Pasquali JL, Soulas-Sprauel P, Korganow AS, Martin T. Auto-reactive B cells in transgenic mice. J Autoimmun. 2007;29:250–256. doi: 10.1016/j.jaut.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowley B, Tang L, Shinton S, Hayakawa K, Hardy RR. Autoreactive B-1 B cells: Constraints on natural autoantibody B cell antigen receptors. J Autoimmun. 2007;29:236–245. doi: 10.1016/j.jaut.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsonis IA, Avrameas S, Moutsopoulos HM. Autoimmunity and pathophysiology. J Autoimmun. 2007;29:203–205. doi: 10.1016/j.jaut.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29:219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]