Summary
Tumor cells display increased metabolic autonomy in comparison to non-transformed cells, taking up nutrients and metabolizing them in pathways that support growth and proliferation. Classical work in tumor cell metabolism focused on bioenergetics, particularly enhanced glycolysis and suppressed oxidative phosphorylation (the ‘Warburg effect’). But the biosynthetic activities required to create daughter cells are equally important for tumor growth, and recent studies are now bringing these pathways into focus. In this review, we discuss how tumor cells achieve high rates of nucleotide and fatty acid synthesis, how oncogenes and tumor suppressors influence these activities, and how glutamine metabolism enables macromolecular synthesis in proliferating cells.
Introduction
Otto Warburg's demonstration that tumor cells rapidly use glucose and convert the majority of it to lactate is still the most fundamental and enduring observation in tumor metabolism [1,2]. His work, which ushered in an era of study on tumor metabolism focused on the relationship between glycolysis and cellular bioenergetics, has been revisited and expanded by generations of tumor biologists. It is now accepted that a high rate of glucose metabolism, exploited clinically by 18FDG-PET scanning, is a metabolic hallmark of rapidly dividing cells, correlates closely with transformation, and accounts for a significant percentage of ATP generated during cell proliferation [3-5,6•,7]. Appreciation of the generality of the Warburg effect stimulated the broader concept that a ‘metabolic transformation’ is required for tumorigenesis. Research over the last few years has reinforced this idea, revealing the conservation of metabolic activities among diverse tumor types, and proving that oncogenic mutations can promote metabolic autonomy by driving nutrient uptake to levels that often exceed those required for cell growth and proliferation [8].
Aerobic glycolysis is just one component of the metabolic transformation. In order to engage in replicative division, a cell must duplicate its genome, proteins and lipids and assemble the components into daughter cells; in short, it must become a factory for macromolecular biosynthesis. These activities require that cells take up extracellular nutrients like glucose and glutamine and allocate them into metabolic pathways that convert them into biosynthetic precursors (Figure 1). Tumor cells can achieve this phenotype through changes in the expression of enzymes that determine metabolic flux rates, including nutrient transporters and enzymes [8-10]. Current studies in tumor metabolism are revealing novel mechanisms for metabolic control, establishing which enzyme isoforms facilitate the tumor metabolic phenotype, and suggesting new targets for cancer therapy.
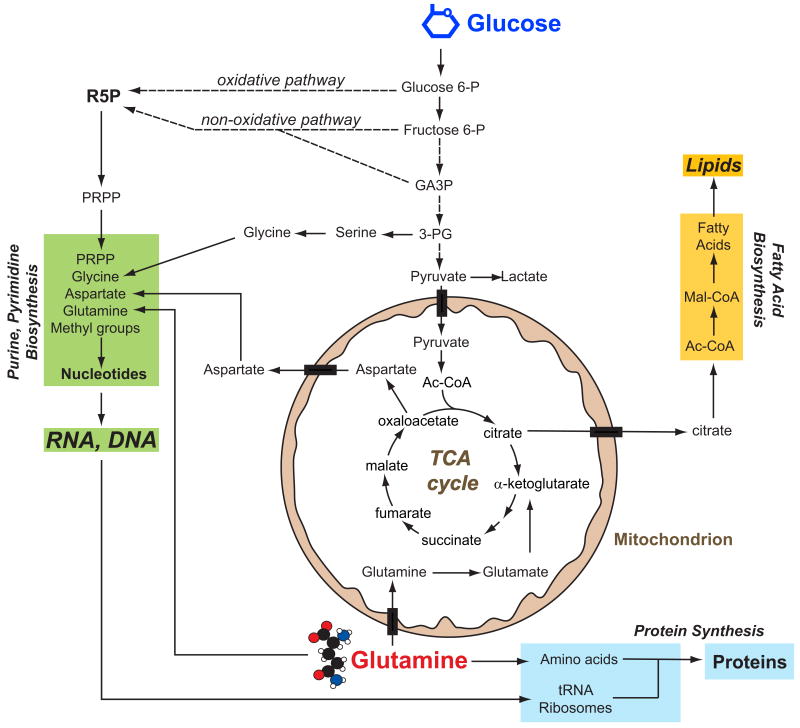
Figure 1. Tumor cells obtain biosynthetic precursors from glucose and glutamine metabolism.
Glucose and glutamine, the two most abundant extracellular nutrients, contribute carbon for the synthesis of the three major classes of macromolecules (nucleic acids, lipids and proteins) in proliferating tumor cells. Biosynthesis of purines and pyrimidines utilizes ribose 5-phosphate (R5P) produced from diversion of glycolytic intermediates into the oxidative and non-oxidative arms of the pentose phosphate pathway, and nonessential amino acids derived from glucose and glutamine. Fatty acid synthesis, used to produce cellular lipids, requires acetyl-CoA (Ac-CoA), most of which is generated from glucose and transferred from the mitochondria to the cytoplasm via citrate. Protein synthesis requires amino acids, tRNAs and ribosomes (proteins and rRNAs). Both glucose and glutamine are used to generate these molecules. In addition to its role as a carbon source, glutamine also donates nitrogen to nucleotide and amino acid synthesis. Abbreviations: P, phosphate; GA3P, glyceraldehyde 3-phosphate; 3-PG, 3-phosphoglycerate; PRPP, phosphoribosyl pyrophosphate; Mal-CoA, malonyl-CoA.
The ongoing challenge in tumor cell metabolism is to understand how individual pathways fit together into the global metabolic phenotype of cell growth. Here we discuss two biosynthetic activities required by proliferating tumor cells: production of ribose-5-phosphate for nucleotide biosynthesis and production of fatty acids for lipid biosynthesis. Nucleotide and lipid biosynthesis share three important characteristics. First, both use glucose as a carbon source. Second, both consume TCA cycle intermediates, imposing the need for a mechanism to replenish the cycle. Third, both require reductive power in the form of NADPH. In this review, we discuss emerging concepts in how proliferating tumor cells achieve high rates of nucleotide and lipid synthesis and propose a model in which glutamine metabolism satisfies crucial aspects of the metabolic transformation, allowing cells to use glucose carbon to build nucleic acid and lipid.
How do tumor cells divert glycolytic carbon towards ribose-5-phosphate synthesis?
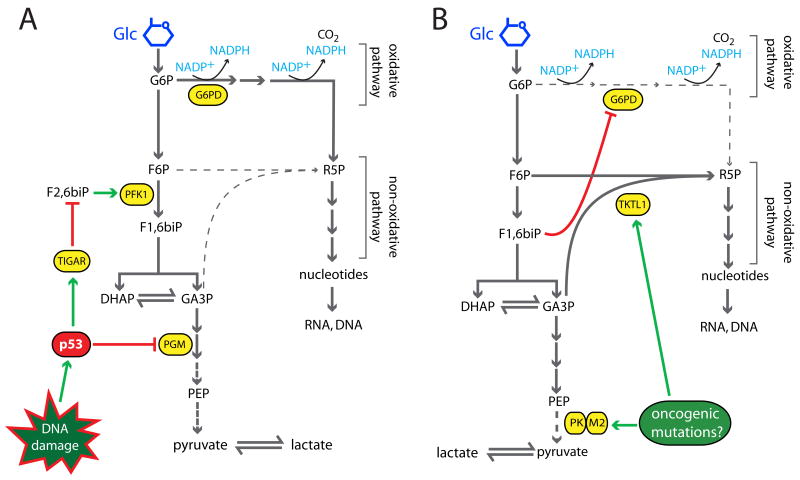
To generate ribose 5-phosphate (R5P) for nucleotide biosynthesis, cells divert carbon from glycolysis into either the oxidative or non-oxidative arm of the pentose phosphate pathway. Oncogenes and tumor suppressors influence both pathways. The p53 target TP53-induced glycolysis and apoptosis regulator (TIGAR) suppresses glycolysis by decreasing levels of the phosphofructokinase-1 activator fructose-2,6-bisphosphate, increasing substrate delivery to the oxidative pathway [11••] (Figure 2A). Enhanced p53-dependent TIGAR expression during genotoxic stress is proposed to increase production of NADPH and R5P to repair DNA damage [12]. p53 also suppresses expression of phosphoglycerate mutase, augmenting diversion of carbon towards R5P [13]. Tumor cells lacking p53 are predicted to lose these effects on the pentose phosphate pathway, causing a relative increase in glycolytic flux. The resulting pyruvate is preferentially converted to lactate due to impaired p53-regulated expression of SCO2, an assembly factor for cytochrome-c oxidase that is required for optimal pyruvate oxidation [14••].
Figure 2. A model for control of oxidative and non-oxidative pentose phosphate flux.
A, Activation of p53 by DNA damage enhances oxidative pentose phosphate flux via effects on TIGAR and PGM. As a result, cells generate NADPH and R5P for nucleotide synthesis and DNA repair. B, Tumors and tumor cell lines, perhaps through the effects of oncogenic mutations, generally express PK-M2 and TKTL1. Dimeric PK-M2 enzyme has sub-maximal activity and allows glycolytic intermediates to accumulate, facilitating TKTL1-catalyzed non-oxidative pentose phosphate flux and suppressing oxidative flux. As a result, there is a mismatch between the amount of R5P and NADPH generated by total pentose phosphate activity. Abbreviations: Glc, glucose; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6biP, fructose 1,6-bisphosphate; F2,6biP, fructose 2,6-bisphosphate; DHAP, dihydroxyacetone phosphate; GA3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; R5P, ribose 5-phosphate; G6PD, glucose 6-phosphate dehydrogenase; PFK1, phosphofructokinase-1; PGM, phosphoglucomutase; TIGAR, TP53-induced glycolysis and apoptosis regulator; PK-M2, pyruvate kinase M2; TKTL1, transketolase-like 1.
How, then, do p53-/- tumor cells achieve high rates of R5P synthesis? Recent data suggest that the distal glycolytic enzyme pyruvate kinase (PK) influences this process [15] (Figure 2B). Humans contain two PK genes (PKLR and PKM2) and four PK isozymes (L, R, M1 and M2). PK-L and PK-R are expressed from alternative PKLR promoters in gluconeogenic tissues and erythrocytes, respectively. PK-M1 and -M2 are coded by alternatively spliced PKM2 transcripts. PK-M1 is confined to the muscle and brain, whereas PK-M2 is found in proliferating cells, including tumor cells of various histological types [16,17] (Figure 2B). PK-M2's effect on glycolysis depends on whether it exists as a highly active tetramer favoring formation of pyruvate and ATP, or a less active dimer, which predominates in tumor cells. In the face of rapid glycolysis, impairment of pyruvate formation by dimeric PK-M2 causes upstream intermediates to accumulate, increasing substrate availability for the non-oxidative pentose phosphate pathway. Accumulation of fructose 1,6-bisphosphate suppresses G6PD, further enhancing non-oxidative flux. Thus, tumor PK-M2 expression predicts a substantial contribution of the non-oxidative pathway to R5P synthesis, and this has been confirmed in several studies [18, 19••,20].
Two other issues emphasize the importance of the non-oxidative pathway in tumor cells. First, several studies have shown that G6PD deficiency, an X-linked condition affecting hundreds of millions of men, does not reduce cancer risk despite a 90% reduction of enzyme activity in many individuals [21,22]. Second, transketolase activity is highly correlated with the rate of tumor growth, and expression of the transketolase isoform TKTL1 in colon, urothelial and ovarian cancers correlates with invasiveness and poor patient outcome [23,24••,25••]. Thus, the widespread expression of PK-M2 and TKTL1 in tumors and the large contribution of the non-oxidative pathway to total nucleotide biosynthesis suggest a state in which the cell's need for R5P outweighs its need for glucose-derived NADPH [26]. This strongly implies that tumor cells engaged in this form of metabolism have a G6PD-independent NADPH supply.
How do tumor cells synthesize fatty acids and lipids?
It has been more than 50 years since the first demonstration that tumors synthesize fatty acids from glucose (Figure 3), and subsequent studies have proven that interrupting fatty acid synthesis can be used as a chemotherapeutic strategy [27]. Tumor cells use fatty acids to modify membrane-targeted proteins and for bulk membrane synthesis, and therefore fatty acid synthesis influences cell signaling and growth. The latter may be critical for tumorigenesis because most acyl groups in tumor lipids appear to result from de novo synthesis rather than import of fatty acids from the extracellular milieu [28,29]. Consistent with a need for robust lipid synthesis, tumor cells express high levels of the lipogenic enzymes ATP-citrate lyase (ACL), acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). All of these have been inhibited in vitro and/or in vivo, resulting in diminished cell proliferation, loss of cell viability, or decreased tumor size [30-34]. In addition to its role in fatty acid synthesis, ACL reinforces the Warburg effect by preventing cytoplasmic accumulation of citrate, which would otherwise suppress glycolysis.
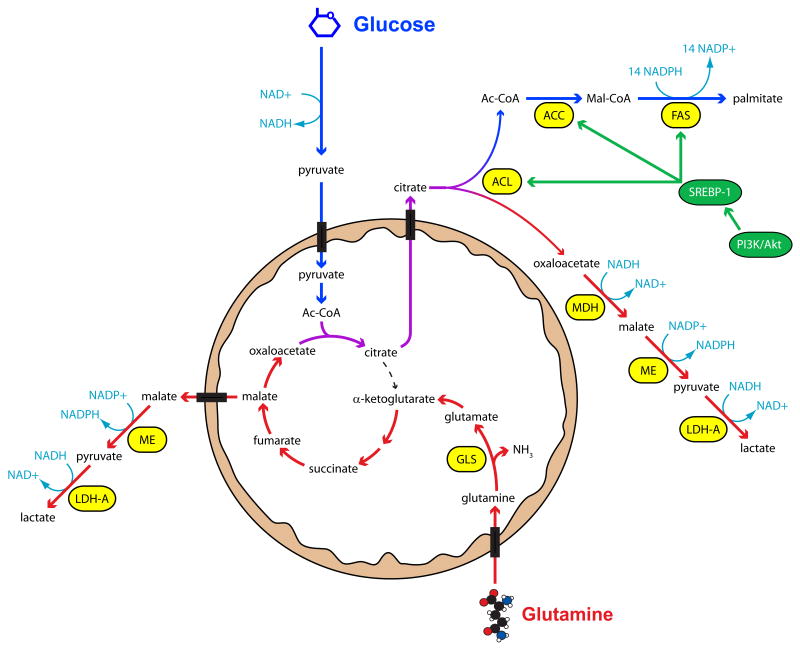
Figure 3. Glutamine metabolism allows tumor cells to sustain TCA cycle activity and produce NADPH during proliferation.
Glucose provides cells with a source of Ac-CoA for fatty acid synthesis (blue arrows), which is enhanced in tumors due to oncogene-driven expression of the lipogenic enzymes ATP-citrate lyase (ACL), acetyl-CoA carboxylase-1 (ACC) and fatty acid synthase (FAS). However, continuous citrate export introduces a deficit to the TCA cycle, and this must be replaced by an anaplerotic flux in order for fatty acid synthesis and cell growth to continue. Metabolism of glutamine (red arrows) provides a mitochondrial oxaloacetate pool for continued citrate synthesis. After citrate cleavage by ACL in the cytoplasm, the resulting oxaloacetate can be converted to malate and ultimately lactate by the low NAD+/NADH ratio created by rapid glycolysis. Glutamine may also be converted to lactate if mitochondrial malate is exported to the cytoplasm and decarboxylated by malic enzyme (ME). This pathway appears to be a major source of NADPH for fatty acid synthesis and other activities in tumor cells. Abbreviations: Ac-CoA, acetyl-CoA; Mal-CoA, malonyl-CoA; MDH, malate dehydrogenase; LDH-A, lactate dehydrogenase-A; GLS, glutaminase; SREBP-1, sterol response element binding protein-1.
Tumors achieve rapid fatty acid synthesis through multiple effects of oncogenic mutations, particularly those involving the phosphatidylinositol 3′kinase (PI3K)/Akt/mTOR pathway (Figure 3). Augmentation of this pathway by mutations activating PI3K or eliminating negative regulators like PTEN comprise a prevalent category of mutation in human cancer [35,36]. The PI3K/Akt/mTOR system stimulates expression of lipogenic genes by increasing nuclear localization of sterol response element binding protein-1 (SREBP-1), a transcription factor whose targets include ACL, ACC and FAS [37,38]. mTOR increases surface expression of glucose transporters, allowing cells to boost import of the major lipogenic precursor [39,40]. Furthermore, PI3K/Akt suppresses β-oxidation, decreasing simultaneous fatty acid synthesis/degradation and maximizing lipid synthesis during proliferation [41,42•].
However, these effects are not sufficient to sustain lipogenesis, because fatty acid synthesis requires two supporting pathways: anaplerosis and NADPH production. The export of mitochondrial citrate must be compensated by replacement of oxaloacetate (OAA) molecules (anaplerosis), or citrate synthesis cannot continue and cells cannot use TCA cycle intermediates in biosynthetic pathways. This important property implies a vital role for anaplerosis in cell growth and tumorigenesis. Aerobic glycolysis, in contrast, does not always reflect cell growth because conditions such as the normoxic stabilization of hypoxia inducible factor-1α (HIF-1α) stimulate aerobic glycolysis while suppressing biosynthetic pathways [43•]. The mechanisms used by tumors to supply anaplerosis are poorly understood. The simplest uses the mitochondrial enzyme pyruvate carboxylase (PC) to generate OAA from pyruvate. However, PC is suppressed in breast carcinoma cells [19••], hepatomas [44,45] and gliomas [46]. An appealing alternative is through glutamine metabolism, as discussed below.
Production of the saturated, 16-carbon fatty acid palmitate requires 14 molecules of NADPH (Figure 3). Two sources of NADPH could contribute to this process: G6PD and cytoplasmic malic enzyme (encoded by the gene ME1). Although the relative importance of these two enzymes has not been extensively studied in tumors, recent reports suggest that malic enzyme flux can be at least as high as G6PD flux [19••,47••]. ME1 is an SREBP-1 target, so tumors with enhanced SREBP-1 activity are likely to have increased ME1 expression [48].
How does glutamine metabolism support biosynthetic activities in tumor cells?
Glutamine metabolism can allow cells to meet both the anaplerotic and NADPH demands of growth. Since the 1950s, it has been clear that tumors consume large amounts of glutamine. The rate of consumption is not explained by protein synthesis because it exceeds the need for essential amino acids by 10-fold [49]. Later studies revealed rapid but partial glutamine oxidation and secretion of glutamine-derived carbon as lactate (Figure 3), establishing glutamine as an energy source in tumor cells [50]. ‘Glutaminolysis,’ the metabolism of glutamine to lactate, is considered a hallmark of tumor cell metabolism [15].
The proximal reactions of glutaminolysis occur in the mitochondria. The first step is catalyzed by phosphate-dependent glutaminases, which deamidate glutamine to form glutamate and ammonia. Interconversion of glutamine and glutamate is typically bidirectional, with glutamine formation catalyzed by glutamine synthetase. In tumors, however, the forward (towards glutamate) reaction is favored by overexpression of glutaminases and/or suppression of glutamine synthetase [51-54]. Thus deamidation is a control point for glutamine metabolism in tumor cells. In xenografts, glutaminase expression is temporally correlated with maximal growth rate, and suppression of glutaminase activity limits tumor growth [55-57].
Little is known about how tumor cells regulate glutaminase expression. Mammals have two major glutaminase activities, K-type (low Km for glutamine, inhibited by glutamate) and L-type (high Km, glutamate resistant). The human K-type enzyme is encoded by the GLS gene, which yields several mRNAs due to alternative polyadenylation and splicing [58,59], and the L-type enzyme is encoded by GLS2. In general, tumor cells have K-type activity, although most cell lines express transcripts from both genes [52]. This suggests that tumors can modulate glutaminase kinetics through relative levels of GLS and GLS2 gene products, resulting in the ability to optimize glutaminase activity despite local fluctuations in glutamine and glutamate concentrations.
In some cells, glutamine-derived α-ketoglutarate (α-KG) is the major source of OAA. A large glutamine-based anaplerotic flux was suggested in rat glioma cells studied with 13C NMR spectroscopy, when adding unlabeled glutamine to cultures containing 13C-glucose suppressed labeling in TCA cycle intermediates [60]. More recently, glutamine deprivation from fibroblasts was shown to reduce cellular pools of TCA cycle intermediates [61]. Finally, NMR spectroscopy in human glioblastoma cells cultured with 13C-labeled glutamine showed conclusively that glutamine contributed the bulk of anaplerotic carbon to the TCA cycle [47••]. The co-existence of robust glucose and glutamine metabolism in these cells resulted in production of citrate molecules containing two glucose-derived carbons (from acetyl-CoA) and four glutamine-derived carbons (from OAA). Further utilization of citrate allowed the glucose-derived carbons to be transferred to fatty acids. Thus, glutamine-based anaplerosis is required in the way some tumor cells use TCA cycle intermediates for growth (Figure 3).
Export of glutamine-derived malate to the cytoplasm short-circuits the TCA cycle but delivers substrate to malic enzyme for NADPH production. Evidence suggests that this can be the major source of NADPH in tumor cells. In human glioblastoma cells, glutaminolysis was predicted to produce more than enough NADPH for fatty acid synthesis; the surplus could presumably be used for nucleotide biosynthesis and maintenance of the glutathione pool [47••]. This was true even though these same cells used glutamine as the major source of anaplerotic carbon. Overall, more than half of the glutamine-derived carbon was secreted as lactate and alanine.
The high rate of lactate and alanine secretion per mole of glutamine, similar to the apparent ‘wastefulness’ of the Warburg effect, has been observed in other proliferating cells [7,50]. In contrast to the common perception that cells use glutamine primarily as a nitrogen source, glutamine metabolism in cancer cells results in excess intracellular nitrogen that must be secreted as alanine or ammonia. Glutaminase removes glutamine's amido group as ammonia. Surprisingly, in glioblastoma cells, the majority of glutamine's amino groups were also lost in α-KG-generating reactions (glutamate dehydrogenase and alanine aminotransferase) [47••]. Therefore, utilization of glutamine as an anaplerotic precursor and source of NADPH results in the secretion of a large fraction of glutamine-derived carbon and nitrogen. Some of the secreted molecules (lactate, alanine) may subsequently be used as precursors for hepatic gluconeogenesis, ultimately providing more fuel for tumor metabolism. At first glance, these appear to be symptoms of metabolic inefficiency, but they may actually reflect a logical and specialized form of metabolism that enables cell growth and proliferation.
Conclusions
An enhanced biosynthetic capacity is a key feature of the metabolic transformation of tumor cells. The activities discussed here (synthesis of nucleotides and fatty acids, consumption of glucose and glutamine) are pervasive among tumors and tumor cell lines. It seems likely that these activities, particularly the use of glutamine as a source of both reductive power and anaplerosis, are general characteristics of cell growth and proliferation. Many questions remain as to how these activities are regulated in tumor cells, including whether they fluctuate according to the stage of the cell cycle. It will be important to determine whether individual oncogenes can regulate all aspects of the metabolic transformation, or if multiple oncogenic mutations exert synergistic effects over tumor cell metabolism.
Acknowledgments
The authors thank members of the Thompson laboratory for critical reading of the manuscript. This work is supported by National Institutes of Health grants PO1 CA104838 (CBT) and K08 DK072565 (RJD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ralph J. DeBerardinis, Email: ralph.deberardinis@utsouthwestern.edu.
Nabil Sayed, Email: sayedn@email.chop.edu.
Dara Ditsworth, Email: darad@mail.med.upenn.edu.
Craig B. Thompson, Email: craig@mail.med.upenn.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Warburg O. Uber den Stoffwechsel der Carcinomzelle. Klin Wochenschr Berl. 1925;4:534–536. [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 4.Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- 5.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. Faseb J. 2004;18:1303–1305. doi: 10.1096/fj.03-1001fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using serial transduction of primary human fibroblasts with telomerase, SV40 large T and small T antigens, and oncogenic H-ras, the authors demonstrated that progressive cellular transformation led to increased reliance on glycolysis for energy production.
- 7.Brand K. Glutamine and glucose metabolism during thymocyte proliferation. Pathways of glutamine and glutamate metabolism. Biochem J. 1985;228:353–361. doi: 10.1042/bj2280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV, Lewis BC, Dolde C, Dang G, Shim H. Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembr. 1997;29:345–354. doi: 10.1023/a:1022446730452. [DOI] [PubMed] [Google Scholar]
- 10.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201–208. [PMC free article] [PubMed] [Google Scholar]
- 11••.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]; The authors identified a p53 target gene, TIGAR, which lowers cellular fructose 2,6-bisphosphate levels, resulting in decreased glycolysis and intracellular reactive oxygen species after exposure to DNA damaging agents. They proposed that TIGAR links p53 activation to cell survival in the face of genotoxic stress via its influence on glucose metabolism.
- 12.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 14••.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]; SCO2 encodes an assembly factor for cytochrome c-oxidase (complex IV of the electron transport chain). The authors determined that SCO2 is a p53 target, and that deletion of p53 results in increased aerobic glycolysis that can be reversed by re-expressing SCO2. Thus, p53 deletion was sufficient to induce the Warburg effect.
- 15.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Netzker R, Greiner E, Eigenbrodt E, Noguchi T, Tanaka T, Brand K. Cell cycle-associated expression of M2-type isozyme of pyruvate kinase in proliferating rat thymocytes. J Biol Chem. 1992;267:6421–6424. [PubMed] [Google Scholar]
- 17.Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Boros LG, Puigjaner J, Cascante M, Lee WN, Brandes JL, Bassilian S, Yusuf FI, Williams RD, Muscarella P, Melvin WS, et al. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57:4242–4248. [PubMed] [Google Scholar]
- 19••.Forbes NS, Meadows AL, Clark DS, Blanch HW. Estradiol stimulates the biosynthetic pathways of breast cancer cells: detection by metabolic flux analysis. Metab Eng. 2006;8:639–652. doi: 10.1016/j.ymben.2006.06.005. [DOI] [PubMed] [Google Scholar]; The authors studied the impact of estradiol stimulation on the metabolism of MCF7 breast cancer cells, using a flux analysis algorithm that allowed them to measure some thirty individual metabolic activities. Estradiol, which induced cell proliferation, concomitantly enhanced glycolysis, the pentose phosphate pathway, glutaminolysis and other activities. The authors concluded that estradiol-stimulated cells consumed glutamine largely to support biosynthesis rather than bioenergetics.
- 20.Boros LG, Cascante M, Lee WN. Metabolic profiling of cell growth and death in cancer: applications in drug discovery. Drug Discov Today. 2002;7:364–372. doi: 10.1016/s1359-6446(02)02179-7. [DOI] [PubMed] [Google Scholar]
- 21.Cocco P, Todde P, Fornera S, Manca MB, Manca P, Sias AR. Mortality in a cohort of men expressing the glucose-6-phosphate dehydrogenase deficiency. Blood. 1998;91:706–709. [PubMed] [Google Scholar]
- 22.Cocco P, Dessi S, Avataneo G, Picchiri G, Heinemann E. Glucose-6-phosphate dehydrogenase deficiency and cancer in a Sardinian male population: a case-control study. Carcinogenesis. 1989;10:813–816. doi: 10.1093/carcin/10.5.813. [DOI] [PubMed] [Google Scholar]
- 23.Comin-Anduix B, Boren J, Martinez S, Moro C, Centelles JJ, Trebukhina R, Petushok N, Lee WN, Boros LG, Cascante M. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur J Biochem. 2001;268:4177–4182. doi: 10.1046/j.1432-1327.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- 24••.Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using immunohistochemistry on more than 1,000 carcinomas covering 16 epithelial tumor entities, the authors detected enhanced TKTL1 expression in more than 80% of the samples. In invasive colon and urothelial carcinomas, TKTL1 staining correlated with poor patient outcome.
- 25••.Krockenberger M, Honig A, Rieger L, Coy JF, Sutterlin M, Kapp M, Horn E, Dietl J, Kammerer U. Transketolase-like 1 expression correlates with subtypes of ovarian cancer and the presence of distant metastases. Int J Gynecol Cancer. 2007;17:101–106. doi: 10.1111/j.1525-1438.2007.00799.x. [DOI] [PubMed] [Google Scholar]; The authors used immunohistochemistry to study TKTL1 expression in a variety of ovarian adenocarcinomas and in normal ovaries. Serous papillary tumors had significantly higher TKTL1 expression than other ovarian carcinomas or normal tissues. Among the twenty serous papillary tumors studied, high TKTL1 expression correlated with poor prognostic parameters, including clinical staging and histopathological grading.
- 26.Stryer L, Berg JM, Tymoczko JL. Biochemistry. 5th. 2002. [Google Scholar]
- 27.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 28.Kannan R, Lyon I, Baker N. Dietary control of lipogenesis in vivo in host tissues and tumors of mice bearing Ehrlich ascites carcinoma. Cancer Res. 1980;40:4606–4611. [PubMed] [Google Scholar]
- 29.Ookhtens M, Kannan R, Lyon I, Baker N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol. 1984;247:R146–153. doi: 10.1152/ajpregu.1984.247.1.R146. [DOI] [PubMed] [Google Scholar]
- 30.Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, Pasternack GR. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- 32.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 34.Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 35.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 36.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 37.Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y, Wang J, Lu X, Thewke DP, Mason RJ. KGF induces lipogenic genes through a PI3K and JNK/SREBP-1 pathway in H292 cells. J Lipid Res. 2005;46:2624–2635. doi: 10.1194/jlr.M500154-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 42•.DeBerardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–37380. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]; Carnitine palmitoyltransferase-1A (CPT1A), the rate-limiting enzyme for β-oxidation of fatty acids, can be allosterically inhibited by malonyl-CoA, and this mechanism regulates β-oxidation activity in muscle and liver. In this study, the authors demonstrated that stimulation of PI3K/Akt suppressed expression of CPT1A, reducing its abundance during cell growth and proliferation. CPT1A suppression was necessary to allow cells to reduce their β-oxidation rate and to achieve maximal rates of lipid synthesis and proliferation.
- 43•.Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors determined that growth factor signaling permitted cells to express HIF-1α, and that HIF-1α controlled the fate of glucose carbon during normoxia as well as hypoxia. Expression of HIF-1α allowed cells to convert a high fraction of glucose carbon into lactate, but suppressed fatty acid synthesis and cell growth. Inhibiting HIF-1α expression with RNA interference increased lipogenesis and cell growth.
- 44.Chang LO, Morris HP. Enzymatic and immunological studies on pyruvate carboxylase in livers and liver tumors. Cancer Res. 1973;33:2034–2041. [PubMed] [Google Scholar]
- 45.Hammond KD, Balinsky D. Activities of key gluconeogenic enzymes and glycogen synthase in rat and human livers, hepatomas, and hepatoma cell cultures. Cancer Res. 1978;38:1317–1322. [PubMed] [Google Scholar]
- 46.Brand A, Engelmann J, Leibfritz D. A 13C NMR study on fluxes into the TCA cycle of neuronal and glial tumor cell lines and primary cells. Biochimie. 1992;74:941–948. doi: 10.1016/0300-9084(92)90078-s. [DOI] [PubMed] [Google Scholar]
- 47••.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors perfused human glioblastoma cells with 13C-labeled glucose and glutamine in order to study metabolic activities during cell proliferation. The cells had a functional TCA cycle characterized by a high rate of anaplerosis, for which almost all the carbon was derived from glutamine. The authors also determined that glutaminolysis could provide more than enough NADPH needed for fatty acid synthesis. The pathways of glutamine utilization resulted in a loss of more than half of glutamine's carbon and nitrogen from the cell.
- 48.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43:1220–1235. [PubMed] [Google Scholar]
- 49.Eagle H, Oyama VI, Levy M, Horton CL, Fleischman R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J Biol Chem. 1956;218:607–616. [PubMed] [Google Scholar]
- 50.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 51.Turner A, McGivan JD. Glutaminase isoform expression in cell lines derived from human colorectal adenomas and carcinomas. Biochem J. 2003;370:403–408. doi: 10.1042/BJ20021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Gomez C, Campos-Sandoval JA, Alonso FJ, Segura JA, Manzanares E, Ruiz-Sanchez P, Gonzalez ME, Marquez J, Mates JM. Co-expression of glutaminase K and L isoenzymes in human tumour cells. Biochem J. 2005;386:535–542. doi: 10.1042/BJ20040996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuno T, Hirai H. Glutamine synthetase and glutaminase activities in various hepatoma cells. Biochem Int. 1989;19:219–225. [PubMed] [Google Scholar]
- 54.Matsuno T, Goto I. Glutaminase and glutamine synthetase activities in human cirrhotic liver and hepatocellular carcinoma. Cancer Res. 1992;52:1192–1194. [PubMed] [Google Scholar]
- 55.Aledo JC, Segura JA, Medina MA, Alonso FJ, Nunez de Castro I, Marquez J. Phosphate-activated glutaminase expression during tumor development. FEBS Lett. 1994;341:39–42. doi: 10.1016/0014-5793(94)80236-x. [DOI] [PubMed] [Google Scholar]
- 56.Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46:243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 57.Lobo C, Ruiz-Bellido MA, Aledo JC, Marquez J, Nunez De Castro I, Alonso FJ. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem J. 2000;348(Pt 2):257–261. [PMC free article] [PubMed] [Google Scholar]
- 58.Elgadi KM, Meguid RA, Qian M, Souba WW, Abcouwer SF. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics. 1999;1:51–62. doi: 10.1152/physiolgenomics.1999.1.2.51. [DOI] [PubMed] [Google Scholar]
- 59.Porter LD, Ibrahim H, Taylor L, Curthoys NP. Complexity and species variation of the kidney-type glutaminase gene. Physiol Genomics. 2002;9:157–166. doi: 10.1152/physiolgenomics.00017.2002. [DOI] [PubMed] [Google Scholar]
- 60.Portais JC, Voisin P, Merle M, Canioni P. Glucose and glutamine metabolism in C6 glioma cells studied by carbon 13 NMR. Biochimie. 1996;78:155–164. doi: 10.1016/0300-9084(96)89500-9. [DOI] [PubMed] [Google Scholar]
- 61.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]