Abstract
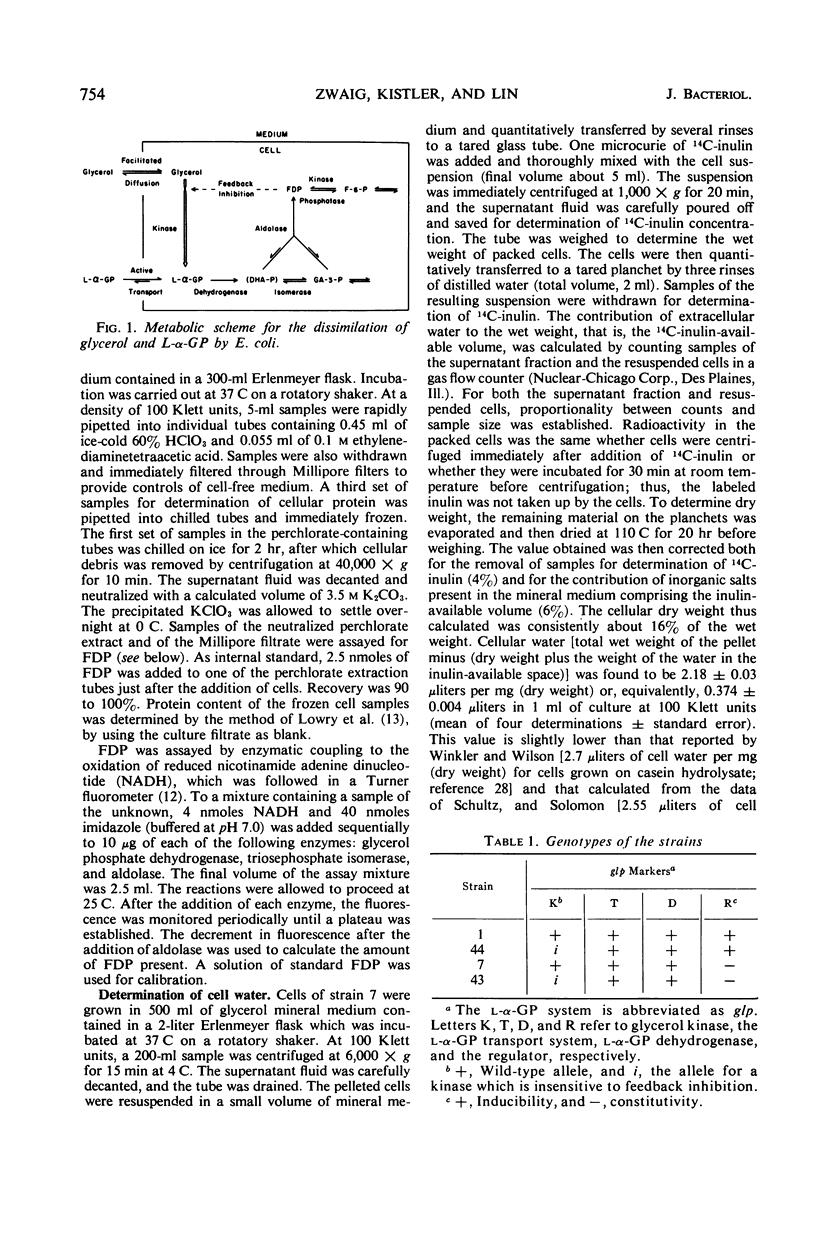
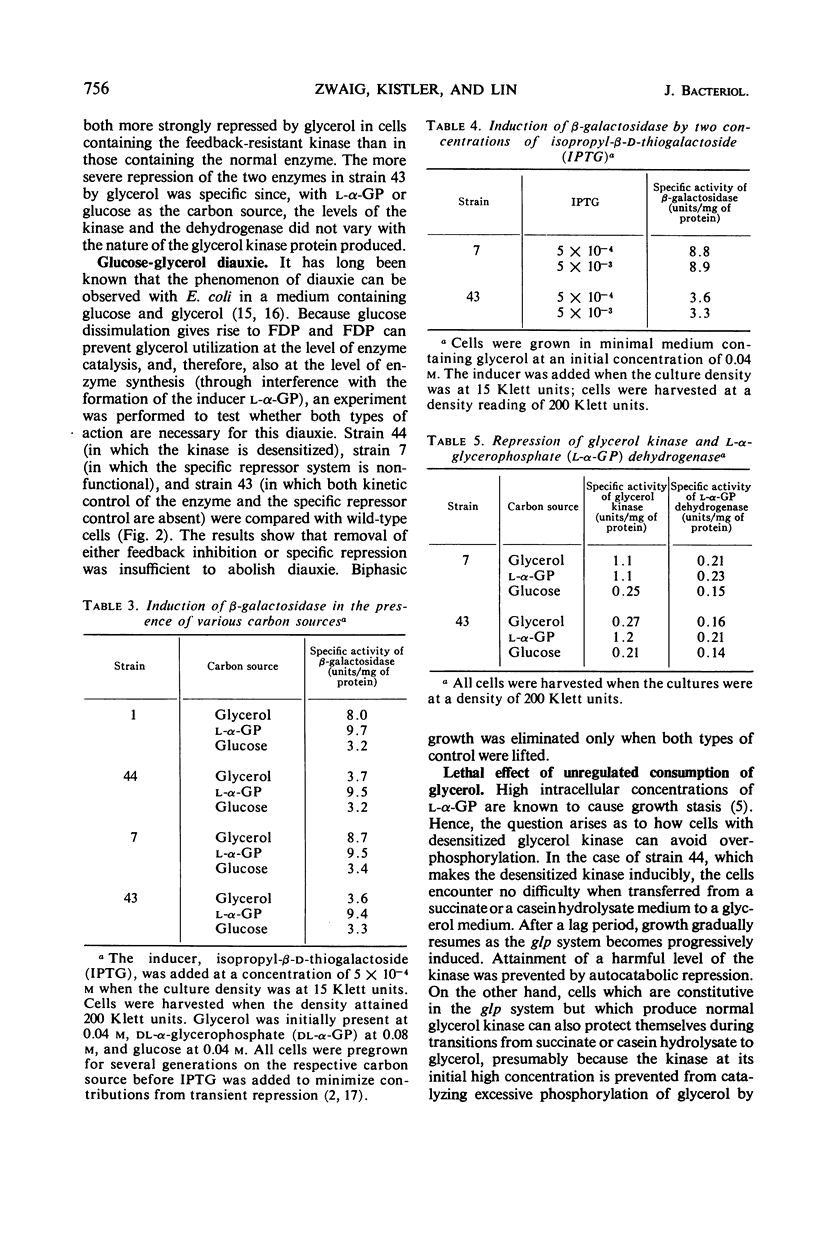
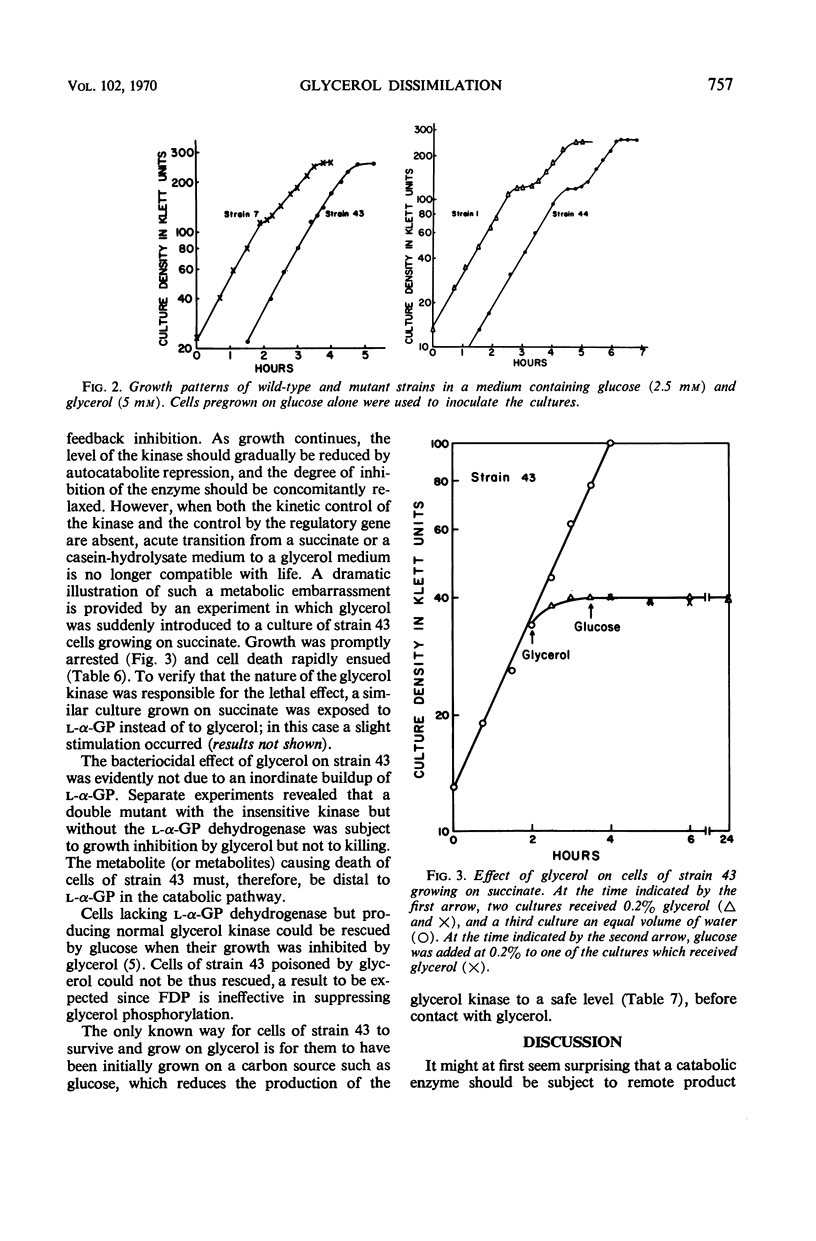
The activity of glycerol kinase is rate-limiting in the metabolism of glycerol by cells of Escherichia coli. A mutant strain producing a glycerol kinase resistant to inhibition by fructose-1,6-diphosphate grows faster than its wild-type parent on glycerol as the sole source of carbon and energy. The amount of intracellular fructose-1,6-diphosphate was determined for wild-type cells growing exponentially on glycerol. The water content of such cells was also determined, allowing calculation of the intracellular concentration of fructose-1,6-diphosphate. This value, 1.7 mm, is adequate to exert substantial inhibition on the wild-type glycerol kinase. The desensitization of glycerol kinase to feedback inhibition also enhances the power of glycerol to exert catabolite repression, both on the enzymes of the glycerol system itself and on those of the lactose system. However, desensitization of glycerol kinase alone does not eliminate the phenomenon of diauxic growth in a glucose-glycerol medium. Biphasic growth in such a medium is abolished if the altered enzyme is produced constitutively. The constitutive production of the mutant kinase at high levels, however, renders the cells vulnerable to glycerol. Thus, when the cells have been grown on a carbon source with a low power for catabolite repression, e.g., succinate, sudden exposure to glycerol leads to overconsumption of the nutrient and cell death.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Properties of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase. J Bacteriol. 1966 Aug;92(2):470–476. doi: 10.1128/jb.92.2.470-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLI-SFORZA L. L. La sessualità nei batteri. Boll Ist Sieroter Milan. 1950 Sep-Oct;29(9-10):281–289. [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959 Nov;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENES G. Glucose repression and the induction of beta-galactosidase synthesis in Escherichia coli. Biochim Biophys Acta. 1961 Jun 24;50:408–409. doi: 10.1016/0006-3002(61)90358-4. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- MOYED H. S., FRIEDMAN M. Interference with feedback control; a mechanism of antimetabolite action. Science. 1959 Apr 10;129(3354):968–969. doi: 10.1126/science.129.3354.968. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- MOYED H. S. Interference with feedback control of enzyme activity. Cold Spring Harb Symp Quant Biol. 1961;26:323–329. doi: 10.1101/sqb.1961.026.01.039. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. Interference with the feed-back control of histidine biosynthesis. J Biol Chem. 1961 Aug;236:2261–2267. [PubMed] [Google Scholar]
- Moses V., Prevost C. Catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem J. 1966 Aug;100(2):336–353. doi: 10.1042/bj1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Effect of mixtures of substrates on the biosynthesis of inducible enzymes in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):260–263. doi: 10.1128/jb.73.2.260-263.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Cation transport in Escherichia coli. I. Intracellular Na and K concentrations and net cation movement. J Gen Physiol. 1961 Nov;45:355–369. doi: 10.1085/jgp.45.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]



