Abstract
The WHO Expert Committee on Biological Standardization authorized the National Institute for Medical Research to establish an International Reference Preparation of Erythropoietin on the basis of the results of an international collaborative assay and to define an international unit with the agreement of the participants.
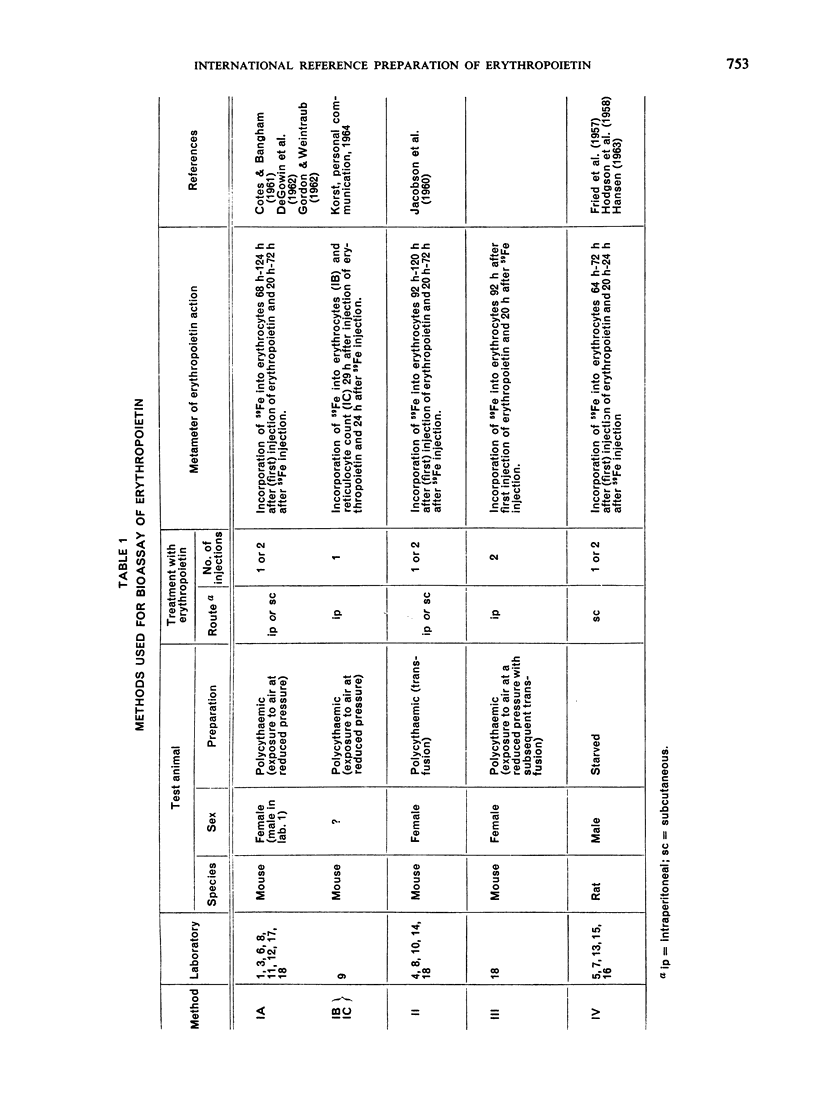
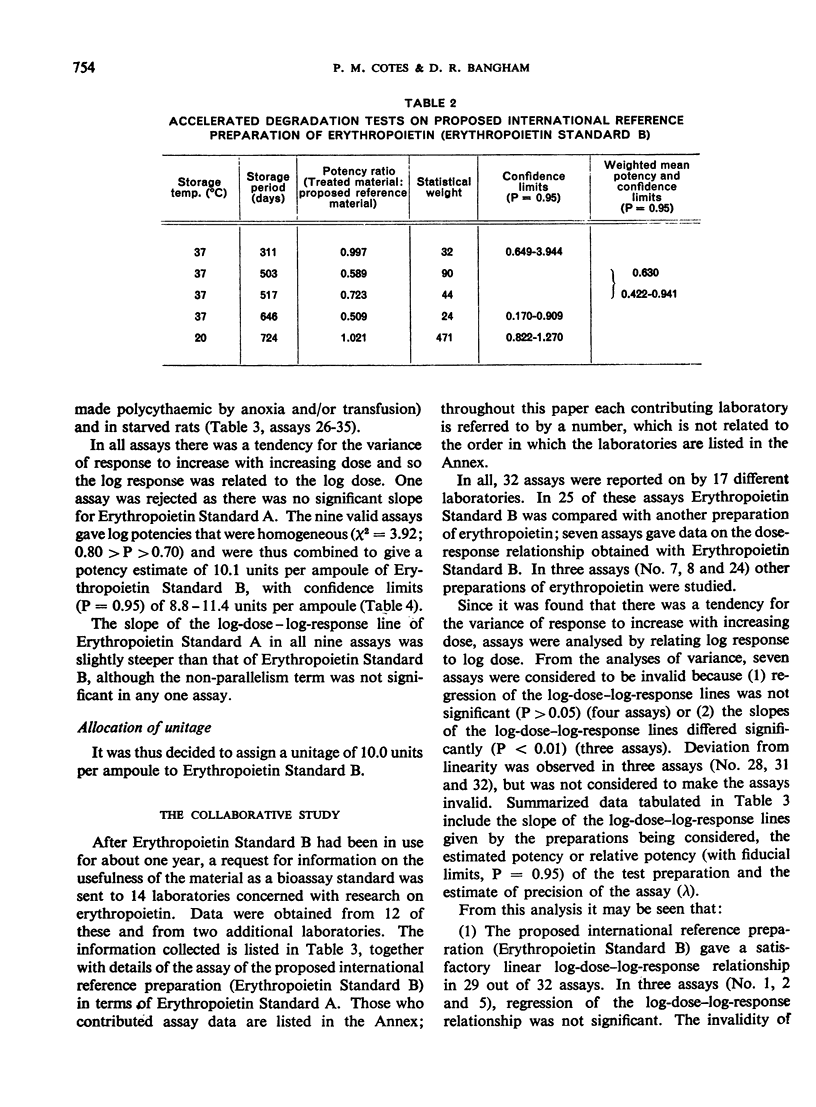
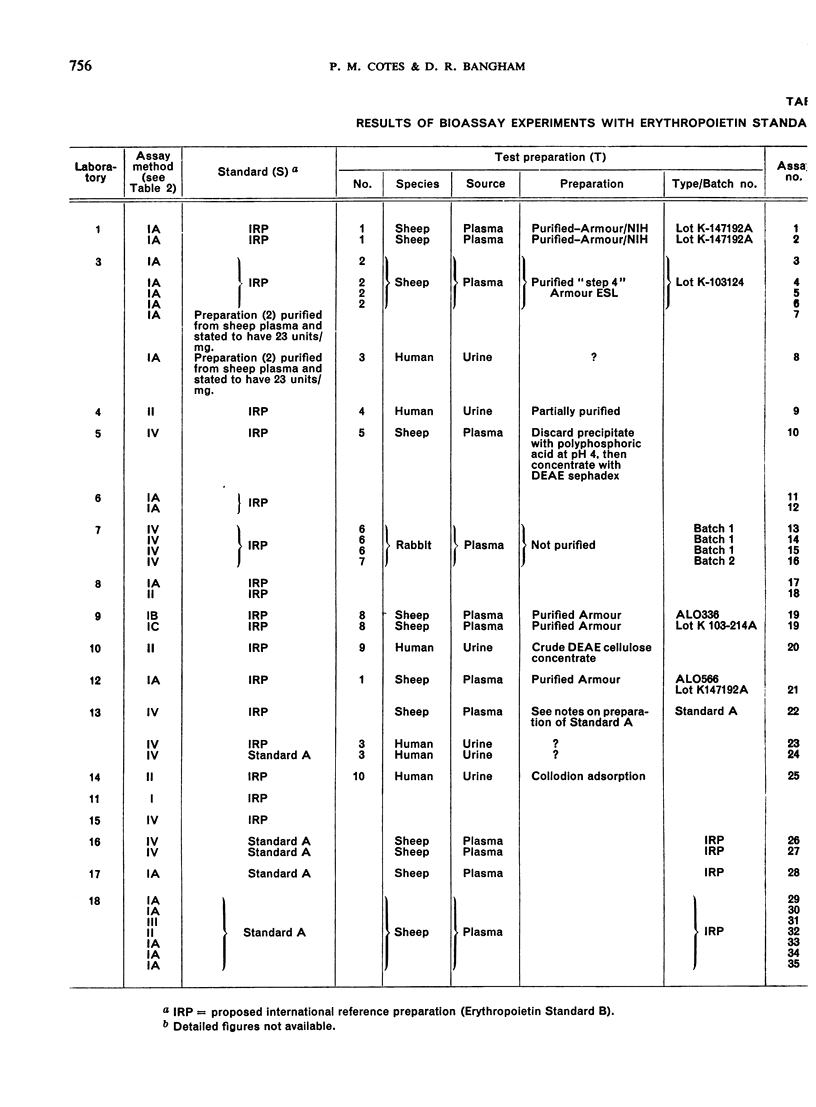
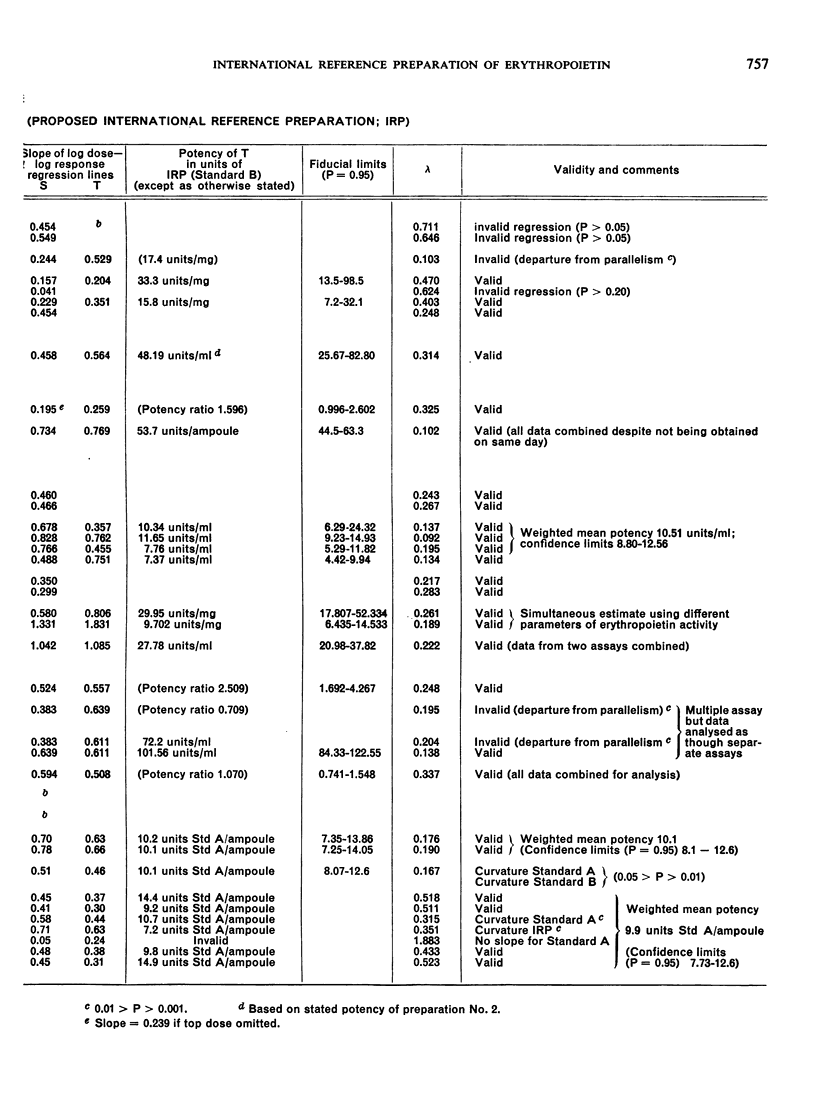
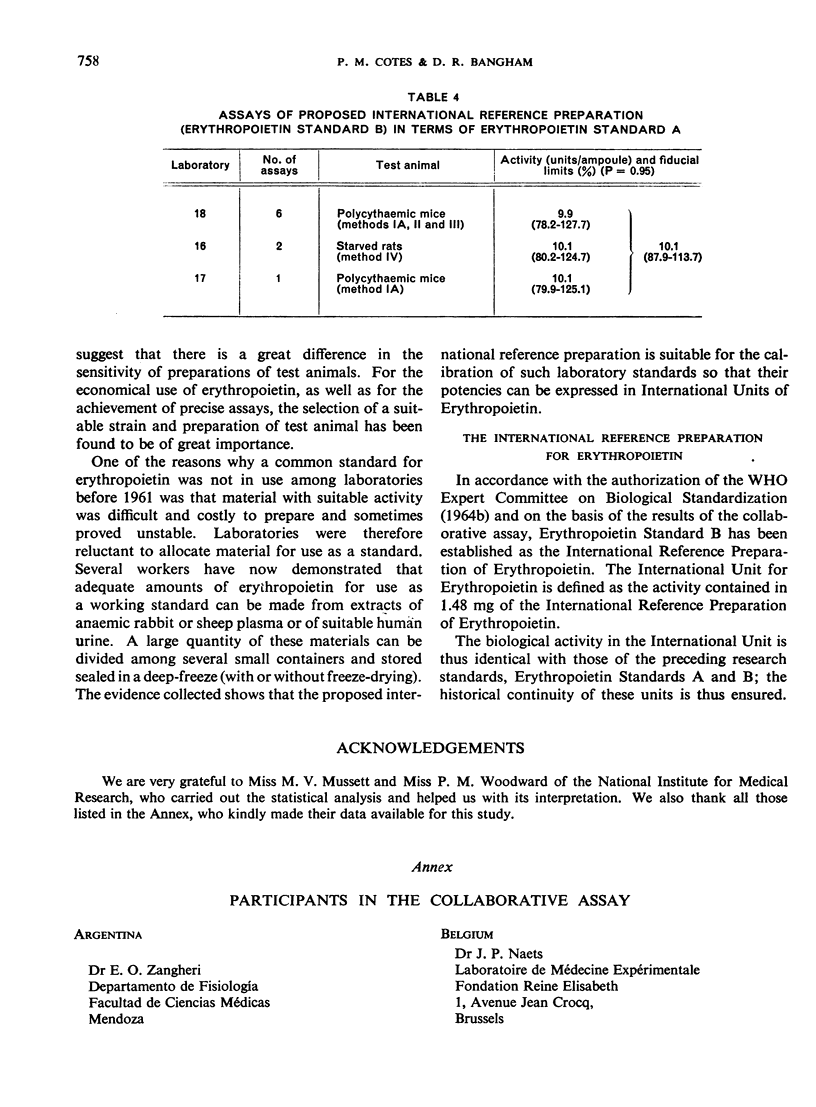
The material investigated was a research standard (Erythropoietin Standard B) that had been in widespread use for some time. The preparation of this material and the estimation of its potency in terms of an earlier research standard (Erythropoietin Standard A) are described. By comparisons with other preparations of erythropoietin, it was found that Erythropoietin Standard B is a suitable standard for the bioassay of many preparations of erythropoietin in current use.
On the basis of the information obtained, Erythropoietin Standard B has been established as the International Reference Preparation of Erythropoietin and the International Unit for Erythropoietin has been defined as the activity contained in 1.48 mg of the International Reference Preparation. This amount has equivalent biological activity to the unit of Erythropoietin Standard A and Standard B and thus ensures the continuity of the units already in widespread use.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTES P. M., BANGHAM D. R. Bio-assay of erythropoietin in mice made polycythaemic by exposure to air at a reduced pressure. Nature. 1961 Sep 9;191:1065–1067. doi: 10.1038/1911065a0. [DOI] [PubMed] [Google Scholar]
- DEGOWIN R. L., HOFSTRA D., GURNEY C. W. The mouse with hypoxia-induced erythremia, an erythropoietin bioassay animal. J Lab Clin Med. 1962 Nov;60:846–852. [PubMed] [Google Scholar]
- FRIED W., GOLDWASSER E., JACOBSON L. O., PLZAK L. F. Studies on erythropoiesis. III. Factors controlling erythropoietin production. Proc Soc Exp Biol Med. 1957 Jan;94(1):237–241. doi: 10.3181/00379727-94-22910. [DOI] [PubMed] [Google Scholar]
- GORDON A. S. Hemopoietine. Physiol Rev. 1959 Jan;39(1):1–40. doi: 10.1152/physrev.1959.39.1.1. [DOI] [PubMed] [Google Scholar]
- HANSEN P. STUDIES ON DETERMINATION OF ERYTHROPOIETIN USING FE59 INCORPORATION IN STARVED RATS. Scand J Clin Lab Invest. 1963;15:357–366. doi: 10.3109/00365516309079756. [DOI] [PubMed] [Google Scholar]
- HODGSON G., PERRETA M., YUDILEVICH D., ESKUCHE I. Assay of hemopoietine in starved animals; properties of urinary hemopoietine. Proc Soc Exp Biol Med. 1958 Oct;99(1):137–142. doi: 10.3181/00379727-99-24274. [DOI] [PubMed] [Google Scholar]
- WEINTRAUB A. H., GORDON A. S., CAMISCOLI J. F. USE OF THE HYPOXIA-INDUCED POLYCYTHEMIC MOUSE IN THE ASSAY AND STANDARDIZATION OF ERYTHROPOIETIN. J Lab Clin Med. 1963 Nov;62:743–752. [PubMed] [Google Scholar]



