Abstract
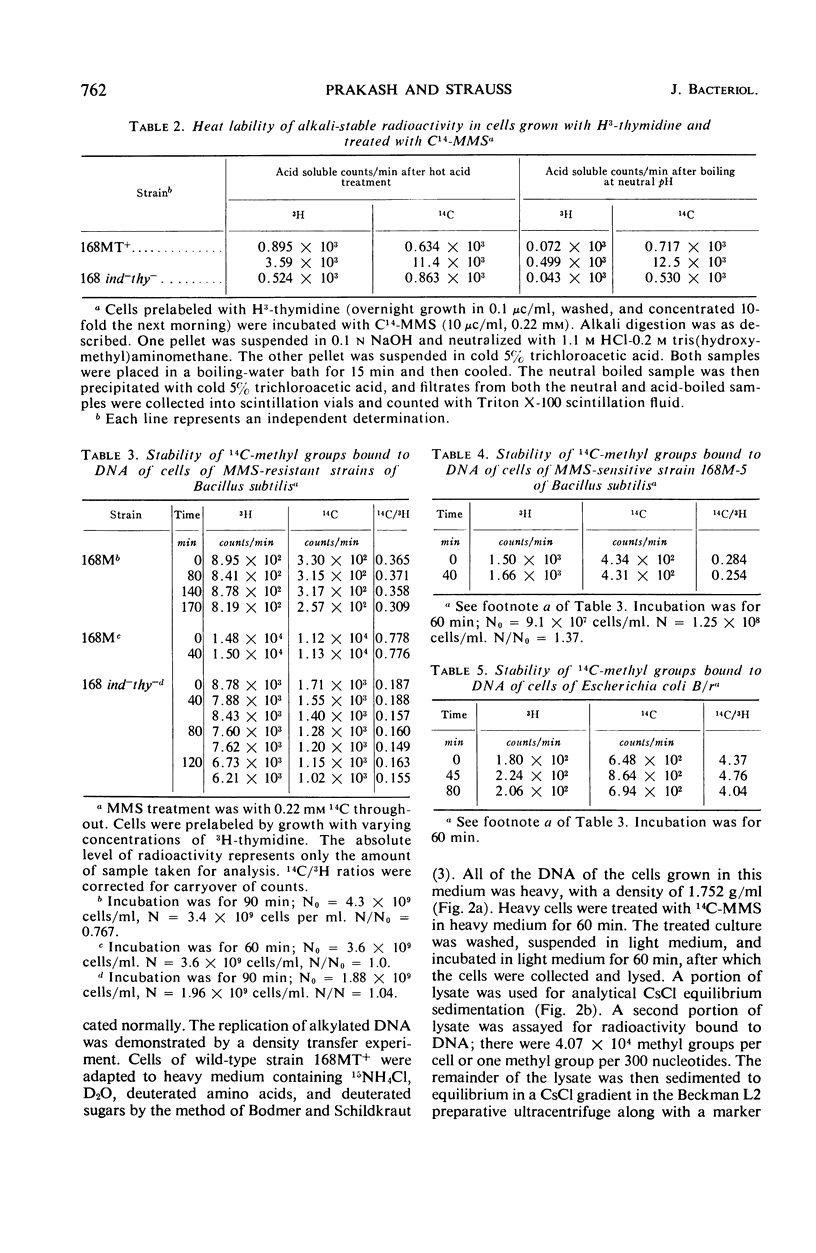
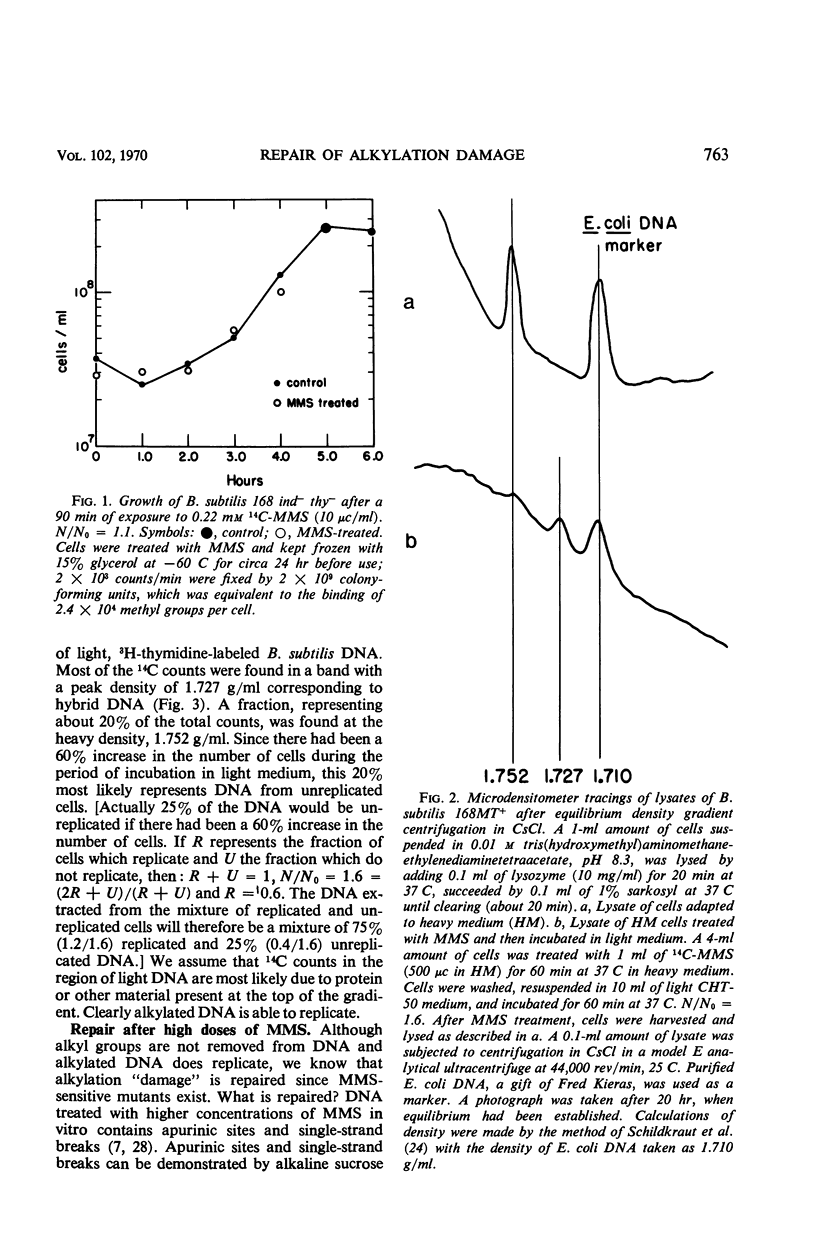
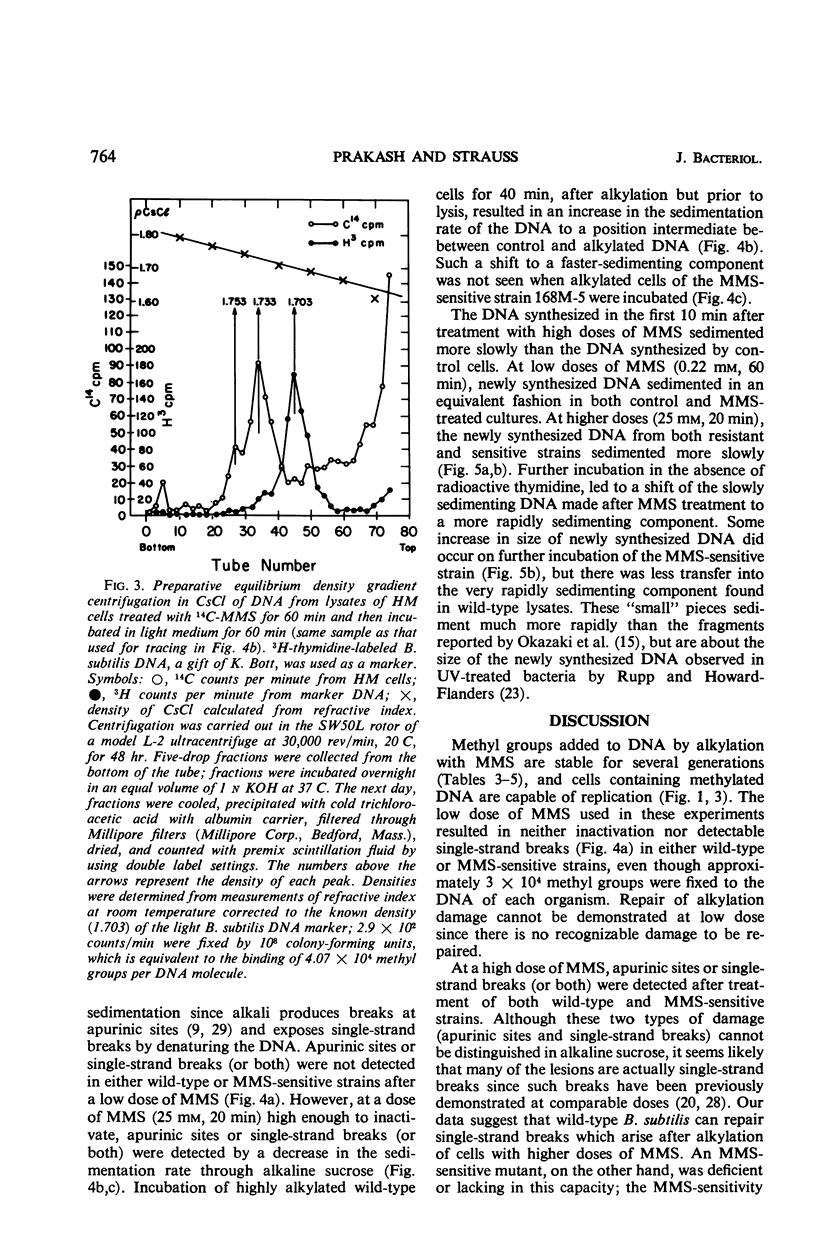
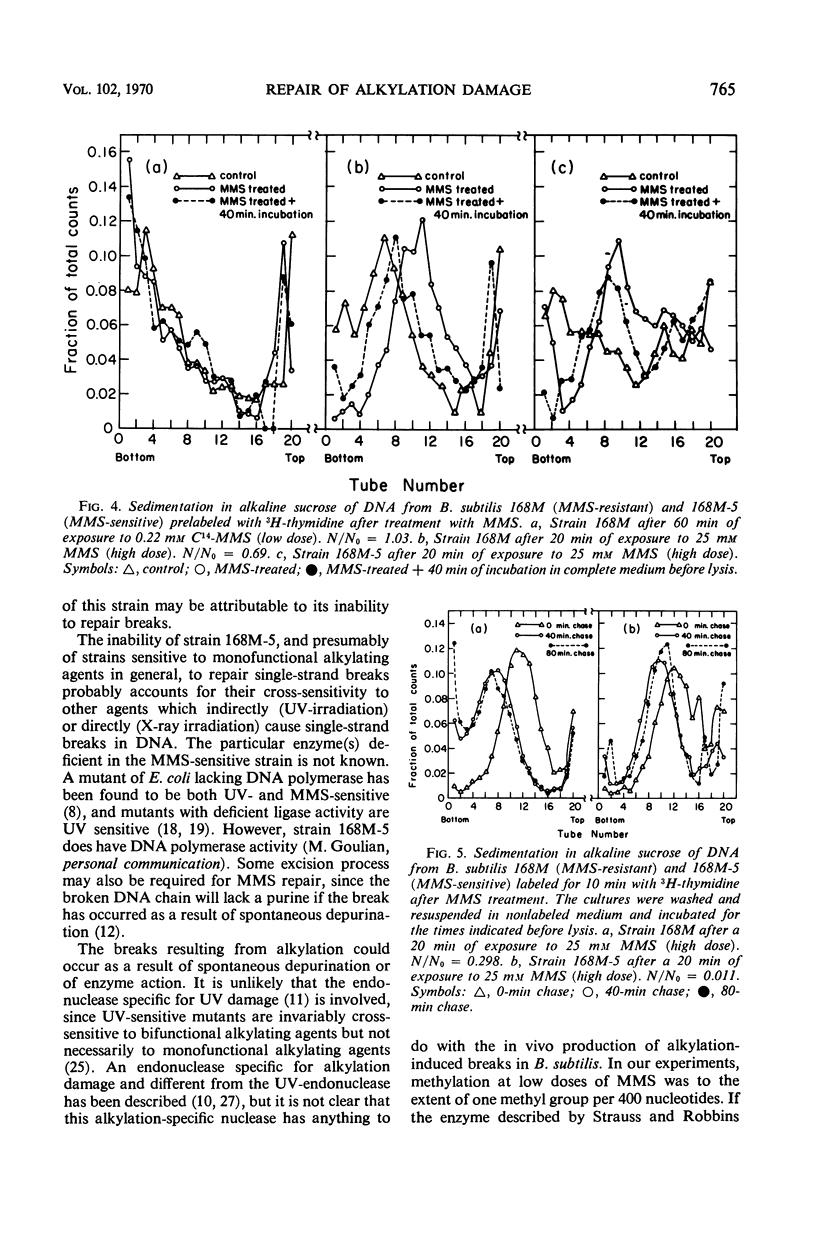
Bacillus subtilis was not inactivated and was able to replicate even though approximately 3 × 104 methyl groups added by methyl methanesulfonate (MMS) were bound to the deoxyribonucleic acid (DNA) of each organism. No significant loss of methyl groups from the DNA occurred for several generations upon incubation of methylated wild-type or MMS-sensitive cells. Single-strand breaks were not observed in the DNA from cells treated at this low MMS dose. Higher doses of MMS resulted in significant killing of both wild-type and MMS-sensitive strains, and the DNA extracted from such treated cells sedimented more slowly than control DNA through alkaline sucrose gradients, indicating the presence of breaks or apurinic sites (or both). These breaks were repaired upon incubation of wild-type but not of MMS-sensitive strains. Repair of damage induced by alkylating agents is probably the repair of breaks which occur as a consequence of high levels of alkylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W., SCHILDKRAUT C. PREPARATION AND CHARACTERIZATION OF 15N2H3H-LABELED DNA FROM BACILLUS SUBTILIS AND ESCHERICHIA COLI PHAGE T2. Anal Biochem. 1964 Jun;8:229–243. doi: 10.1016/0003-2697(64)90051-x. [DOI] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EFFECTS OF ALKYLATING AGENTS ON T2 AND T4 BACTERIOPHAGES. Biochem J. 1963 Oct;89:138–144. doi: 10.1042/bj0890138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassa G. Biochemical genetics of bacterial sporulation. I. Unidirectional pleiotropic interactions among genes controlling sporulation in Bacillus subtilis. Mol Gen Genet. 1969;104(1):73–103. [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Uretz R. B. Mechanism of photoinactivation of coliphage T-7 sensitized by acridine orange. Virology. 1966 Sep;30(1):97–103. doi: 10.1016/s0042-6822(66)81013-9. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D. Effects of some chemical mutagens and carcinogens on nucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:89–131. doi: 10.1016/s0079-6603(08)60232-9. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Zimm B. H. Molecular weight of the DNA in the chromosomes of E. coli and B. subtilis. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1636–1641. doi: 10.1073/pnas.54.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. O. Estimation of the products of DNA alkylation. J Chromatogr. 1968 Jun 4;35(2):292–294. doi: 10.1016/s0021-9673(01)82387-6. [DOI] [PubMed] [Google Scholar]
- Olson A. O., McCalla D. R. Excision of 7-methylguanine from the DNA of Euglena gracilis. Biochim Biophys Acta. 1969 Jul 22;186(1):229–231. doi: 10.1016/0005-2787(69)90509-7. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive, radiation-sensitive mutant of Escherichia coli. II. DNA replication. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1195–1202. doi: 10.1073/pnas.64.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Strauss B., Robbins M., Marone R. Nature of the repair of methyl methanesulfonate-induced damage in Bacillus subtilis. J Bacteriol. 1967 Mar;93(3):1056–1062. doi: 10.1128/jb.93.3.1056-1062.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. IV. Reactions of oligodeoxynucleotides with monofunctional alkylating agents leading to backbone breakage. Biochim Biophys Acta. 1969 Oct 22;190(2):418–433. doi: 10.1016/0005-2787(69)90091-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Crathorn A. R., Brent T. P. Repair of alkylated DNA in mammalian cells. Nature. 1968 Jun 8;218(5145):970–972. doi: 10.1038/218970a0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Searashi T., Strauss B. Relation of the repair of damage induced by a monofunctional alkylating agent to the repair of damage induced by ultraviolet light in Bacillus subtilis. Biochem Biophys Res Commun. 1965 Sep 22;20(6):680–687. doi: 10.1016/0006-291x(65)90069-0. [DOI] [PubMed] [Google Scholar]
- Strauss B. S. DNA repair mechanisms and their relation to mutation and recombination. Curr Top Microbiol Immunol. 1968;44:1–85. [PubMed] [Google Scholar]
- Strauss B. S., Robbins M. DNA methylated in vitro by a monofunctional alkylating agent as a substrate for a specific nuclease from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Jun 18;161(1):68–75. doi: 10.1016/0005-2787(68)90295-5. [DOI] [PubMed] [Google Scholar]
- TAMM C., SHAPIRO H. S., LIPSHITZ R., CHARGAFF E. Distribution density of nucleotides within a desoxyribonucleic acid chain. J Biol Chem. 1953 Aug;203(2):673–688. [PubMed] [Google Scholar]
- TSUBOI K. K., PRICE T. D. Isolation, detection and measure of microgram quantities of labeled tissue nucleotides. Arch Biochem Biophys. 1959 Mar;81(1):223–237. doi: 10.1016/0003-9861(59)90192-4. [DOI] [PubMed] [Google Scholar]



