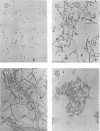
Abstract
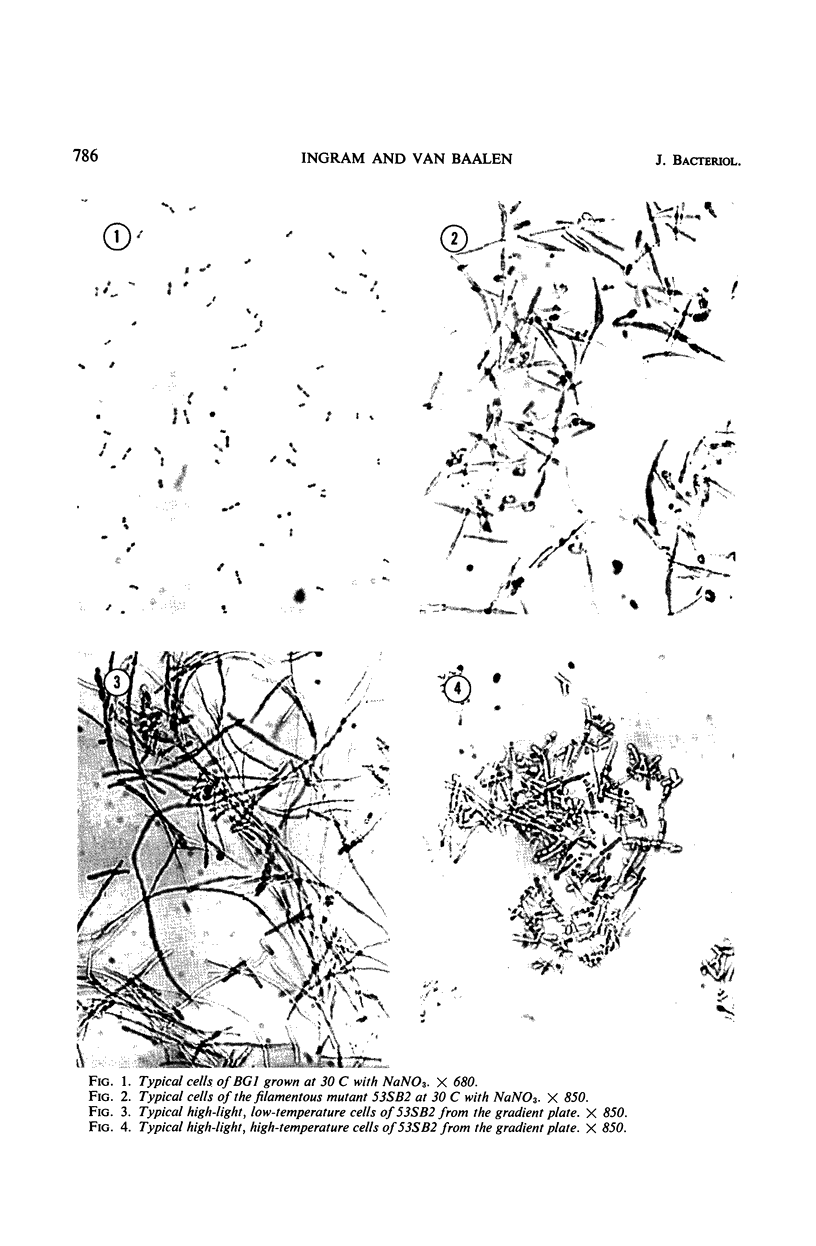
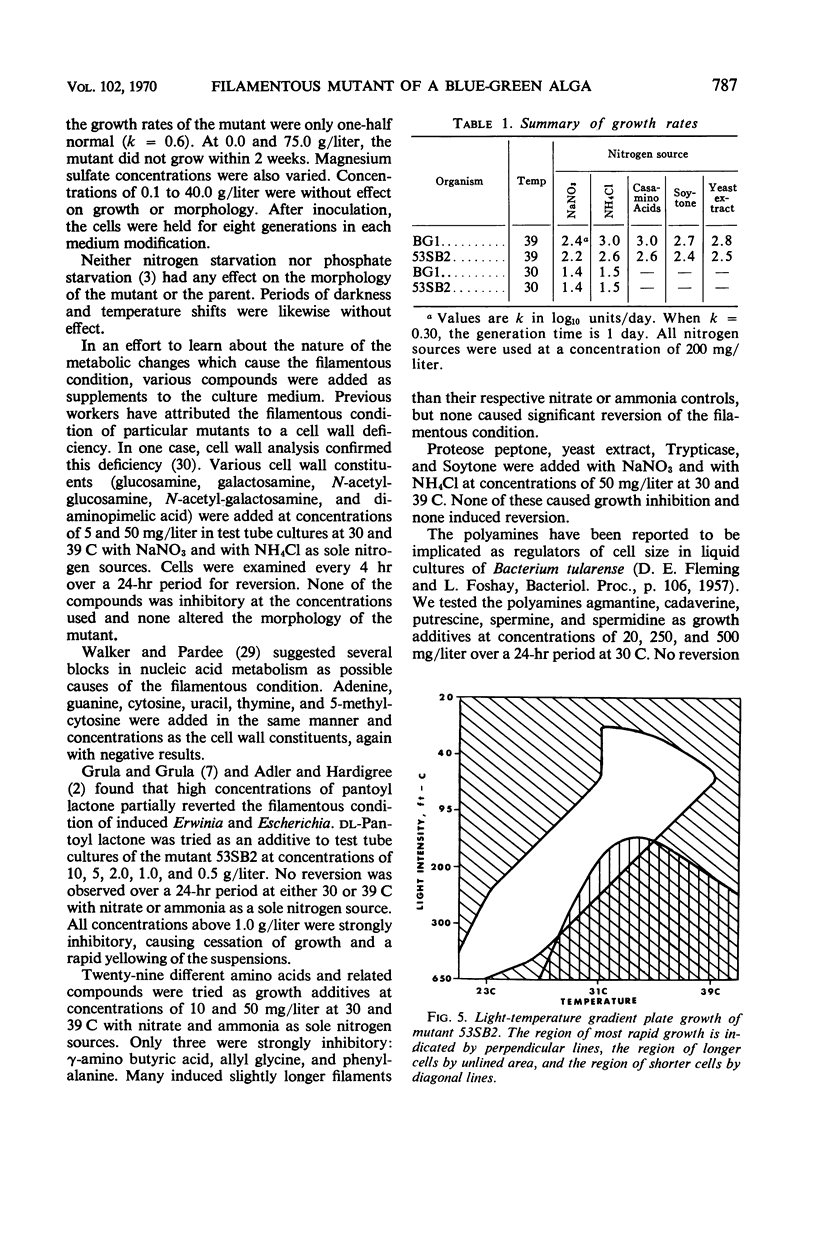
Filamentous mutants were induced in a coccoid blue-green alga, Agmenellum quadruplicatum strain BG1, after treatment with N-methyl-N′-nitro-N-nitrosoguanidine (NTG). The mutants fall into two general classes: filaments with cross walls and filaments without cross walls. All mutants of these general types derived from BG1 are stable and have growth rates the same as or very similar to the wild type under a variety of conditions. Detailed examination of one mutant, 53SB2, revealed no difference in deoxyribonucleic acid content nor in base ratios. Mutant 53SB2 did not revert to the normal cell size and shape when grown under different physical conditions nor upon the addition of potential reversing agents to the basal medium. It is our general experience that filamentous mutants such as those described here in BG1 are commonly induced in other coccoid blue-green algae after NTG treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Cell elongation in strains of Escherichia coli. J Bacteriol. 1964 May;87(5):1240–1242. doi: 10.1128/jb.87.5.1240-1242.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterton J. C., Van Baalen C. Phosphorus deficiency and phosphate uptake in the blue-green alga Anacystis nidulans. Can J Microbiol. 1968 Apr;14(4):341–348. doi: 10.1139/m68-056. [DOI] [PubMed] [Google Scholar]
- CASSEL W. A., HUTCHINSON W. G. Nuclear studies on the smaller Myxophyceae. Exp Cell Res. 1954 Feb;6(1):134–150. doi: 10.1016/0014-4827(54)90155-x. [DOI] [PubMed] [Google Scholar]
- Craig I. W., Leach C. K., Carr N. G. Studies with deoxyribonucleic acid from blue-green algae. Arch Mikrobiol. 1969;65(3):218–227. doi: 10.1007/BF00407105. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldal P., French C. S. Algal Growth in Crossed Gradients of Light Intensity and Temperature. Plant Physiol. 1958 Jul;33(4):249–252. doi: 10.1104/pp.33.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968 Feb;95(2):520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. IV. Cytochemical changes. J Bacteriol. 1967 Jan;93(1):367–378. doi: 10.1128/jb.93.1.367-378.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K. Induction of pleomorphism in Lactobacillus bifidus. J Bacteriol. 1968 Feb;95(2):710–711. doi: 10.1128/jb.95.2.710-711.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H. D. Action of mutagenic chemicals on Anacystis nidulans. 3. N-methyl-N'-Nitro-N-nitrosoguanidine. Arch Mikrobiol. 1968;63(1):95–102. doi: 10.1007/BF00407068. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Gross J. D. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J Bacteriol. 1967 Nov;94(5):1603–1608. doi: 10.1128/jb.94.5.1603-1608.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROVASOLI L., MCLAUGHLIN J. J., DROOP M. R. The development of artificial media for marine algae. Arch Mikrobiol. 1957;25(4):392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M. Short-trichome mutant of Plectonema boryanum. J Bacteriol. 1969 Feb;97(2):975–976. doi: 10.1128/jb.97.2.975-976.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders P. P., Schultz G. A., Saunders G. F. Effect of 5-fluorouracil on the growth and morphology of a polyauxotrophic strain of Bacillus subtilis. J Bacteriol. 1968 Aug;96(2):560–562. doi: 10.1128/jb.96.2.560-562.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Cellular division of penicillin-induced filaments of Escherichia coli. Folia Microbiol (Praha) 1967;12(3):240–247. doi: 10.1007/BF02868738. [DOI] [PubMed] [Google Scholar]
- VAN DE PUTTE P., VAN DILLEWIJN, ROERSCH A. THE SELECTION OF MUTANTS OF ESCHERICHIA COLI WITH IMPAIRED CELL DIVISION AT ELEVATED TEMPERATURE. Mutat Res. 1964 Jul;106:121–128. doi: 10.1016/0027-5107(64)90014-4. [DOI] [PubMed] [Google Scholar]
- Van Baalen C. Mutation of the Blue-Green Alga, Anacystis nidulans. Science. 1965 Jul 2;149(3679):70–70. doi: 10.1126/science.149.3679.70. [DOI] [PubMed] [Google Scholar]
- Van Baalen C. The effects of ultraviolet irradiation on a coccoid blue-green alga: survival, photosynthesis, and photoreactivation. Plant Physiol. 1968 Oct;43(10):1689–1695. doi: 10.1104/pp.43.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G. Characteristics of cell walls from morphological variants of Escherichia coli. J Gen Microbiol. 1966 Jan;42(1):83–92. doi: 10.1099/00221287-42-1-83. [DOI] [PubMed] [Google Scholar]