Abstract
Early and persistent antibody is needed for maximum protection to be afforded to persons severely exposed to rabies. With vaccine alone, detectable antibody production takes 7-10 days, and the passive antibody introduced with antirabies serum or gamma-globulin is commonly used to fill this gap. However, administration of antirabies serum or gamma-globulin with vaccine interferes with the antigenic action of the vaccine.
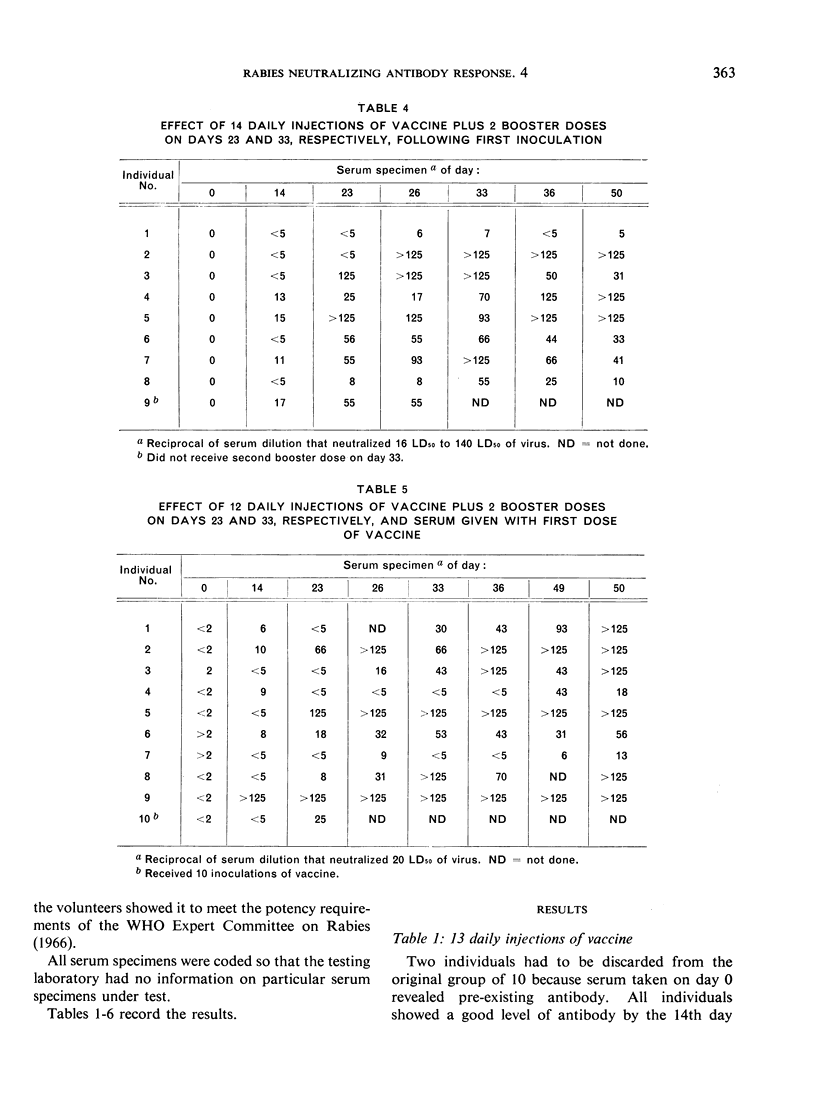
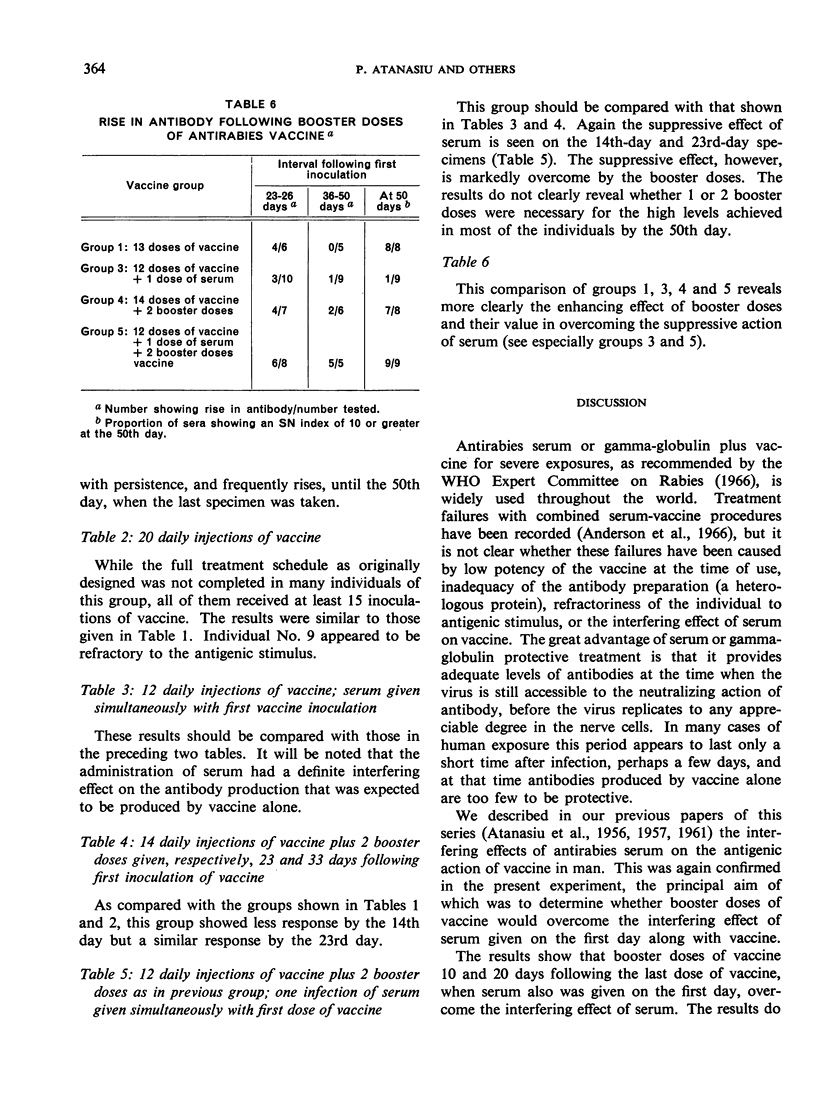
The studies reported in this paper provide further evidence that the interfering effect of serum can be overcome by giving booster doses of vaccine, as recommended by the WHO Expert Committee on Rabies, 10 and 20 days after the end of the usual 12- to 14-dose series of vaccine inoculations. While the results do not clearly indicate whether a single booster dose might be sufficient, the authors consider the second booster useful to ensure both degree and length of antibody duration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATANASIU P., BAHMANYAR M., BALTAZARD M., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOMAROV A., KOPROWSKI H., LEPINE P. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. II. Bull World Health Organ. 1957;17(6):911–932. [PMC free article] [PubMed] [Google Scholar]
- ATANASIU P., BAHMANYAR M., BALTAZARD M., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOMAROV A., KOPROWSKI H., LEPINE P. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. Bull World Health Organ. 1956;14(4):593–611. [PMC free article] [PubMed] [Google Scholar]
- ATANASIU P., CANNON D. A., DEAN D. J., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOPROWSKI H., LEPINE P., PEREZ GALLARDO F. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. 3. Bull World Health Organ. 1961;25:103–114. [PMC free article] [PubMed] [Google Scholar]
- Anderson J. A., Daly F. T., Jr, Kidd J. C. Human rabies after antiserum and vaccine postexposure treatment. Case report and review. Ann Intern Med. 1966 Jun;64(6):1297–1302. doi: 10.7326/0003-4819-64-6-1297. [DOI] [PubMed] [Google Scholar]
- Habel K. Laboratory techniques in rabies. Habel test for potency. Monogr Ser World Health Organ. 1966;23:140–143. [PubMed] [Google Scholar]
- Seligmann E. B., Jr Laboratory techniques in rabies. Potency-test requirements of the United States National Institutes of Health (NIH). Monogr Ser World Health Organ. 1966;23:145–151. [PubMed] [Google Scholar]


