Abstract
Coevolution of viruses and the host cells occurred in BHK-21 cell cultures persistently infected with foot-and-mouth disease virus (FMDV) (J. C. de la Torre, E. Martínez-Salas, J. Diez, A. Villaverde, F. Gebauer, E. Rocha, M. Dávila, and E. Domingo, J. Virol. 62:2050-2058, 1988). In the present report we provide evidence of an extreme phenotypic heterogeneity of the cells, which was generated in the course of persistence. A total of 248 stable cell clones isolated from FMDV carrier cultures at early or late passages were analyzed. At least six distinct cell phenotypes were distinguished with regard to cell morphology, resistance to FMDV strain C-S8c1, and cell growth characteristics. No infectious FMDV or viral RNA was detected in variant cell clones, suggesting that the altered phenotypes were caused by inheritable cell modifications, selected in the course of persistence. Thus, the FMDV-BHK-21 carrier cell system must be described as a dynamic interaction between an evolving heterogeneous population of virus and multiple cell variants. We suggest that cell heterogeneity confers a selective advantage for long-term virus and cell survival by providing the cell population with a range of responses toward FMDV.
Full text
PDF
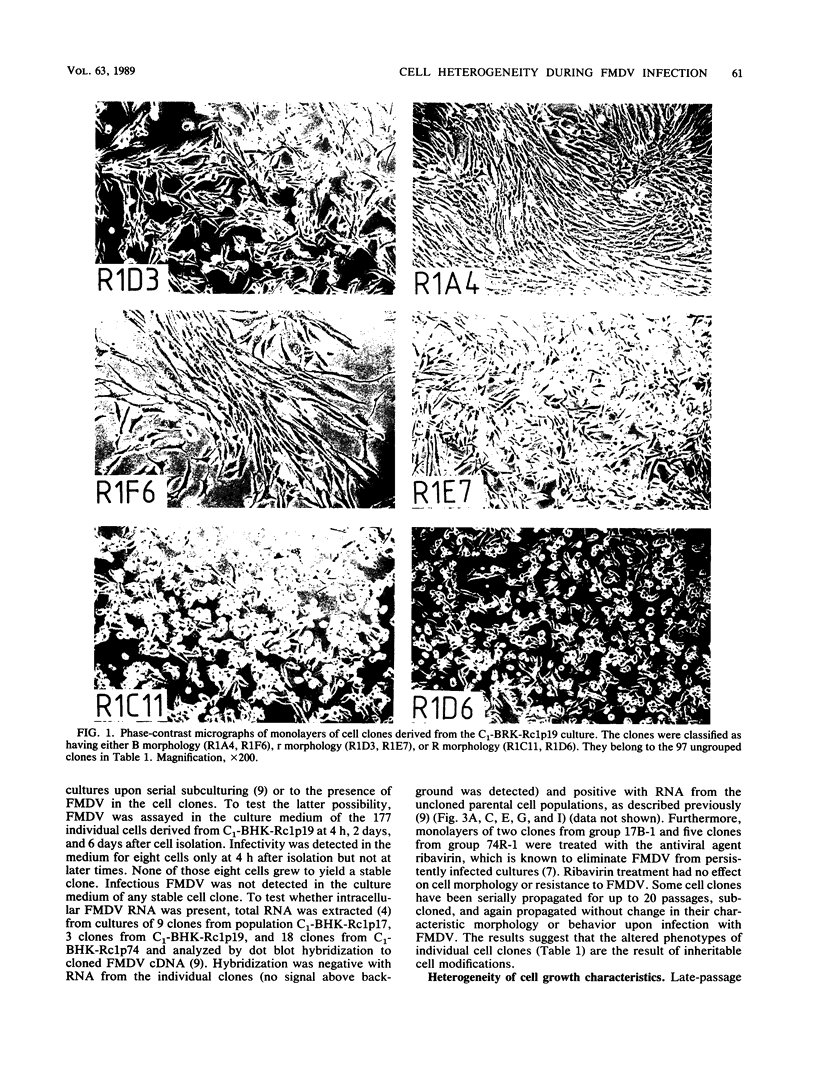

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L. Foot-and-mouth disease. Annu Rev Microbiol. 1968;22:201–244. doi: 10.1146/annurev.mi.22.100168.001221. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Cifone M. A., Fidler I. J. Increasing metastatic potential is associated with increasing genetic instability of clones isolated from murine neoplasms. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6949–6952. doi: 10.1073/pnas.78.11.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E. C., Stone K. R., Bloch M., McDivitt R. W. Heterogeneity in the production of collagens and fibronectin by morphologically distinct clones of a human tumor cell line: evidence for intratumoral diversity in matrix protein biosynthesis. Cancer Res. 1987 Nov 15;47(22):6086–6092. [PubMed] [Google Scholar]
- Gebauer F., de la Torre J. C., Gomes I., Mateu M. G., Barahona H., Tiraboschi B., Bergmann I., de Mello P. A., Domingo E. Rapid selection of genetic and antigenic variants of foot-and-mouth disease virus during persistence in cattle. J Virol. 1988 Jun;62(6):2041–2049. doi: 10.1128/jvi.62.6.2041-2049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan M. S., Kramerov D. A., Tulchinsky E. M., Revasova E. S., Lukanidin E. M. Activation of putative transposition intermediate formation in tumor cells. EMBO J. 1985 Sep;4(9):2209–2215. doi: 10.1002/j.1460-2075.1985.tb03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger R. S. The isolation and characterization of foot-and-mouth disease virus from clinically normal herds of cattle in Botswana. J Hyg (Lond) 1968 Mar;66(1):27–36. doi: 10.1017/s0022172400040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram V. M., Ogren M. P., Chatot C. L., Gossels J. M., Owens B. B. Diversity among Purkinje cells in the monkey cerebellum. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7131–7135. doi: 10.1073/pnas.82.20.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N. Diversity achieved by diverse mechanisms: gene conversion in developing B cells of the chicken. Cell. 1987 Feb 13;48(3):359–360. doi: 10.1016/0092-8674(87)90439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- O'Brien R. L., Brinster R. L., Storb U. Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice. 1987 Mar 26-Apr 1Nature. 326(6111):405–409. doi: 10.1038/326405a0. [DOI] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Miller J. B., Stockdale F. E. Cell diversification within the myogenic lineage: in vitro generation of two types of myoblasts from a single myogenic progenitor cell. Cell. 1987 Feb 27;48(4):659–670. doi: 10.1016/0092-8674(87)90244-3. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Sutmoller P., Gaggero A. Foot-and mouth diseases carriers. Vet Rec. 1965 Aug 14;77(33):968–969. doi: 10.1136/vr.77.33.968. [DOI] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Alarcón B., Martínez-Salas E., Carrasco L., Domingo E. Ribavirin cures cells of a persistent infection with foot-and-mouth disease virus in vitro. J Virol. 1987 Jan;61(1):233–235. doi: 10.1128/jvi.61.1.233-235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Dávila M., Sobrino F., Ortín J., Domingo E. Establishment of cell lines persistently infected with foot-and-mouth disease virus. Virology. 1985 Aug;145(1):24–35. doi: 10.1016/0042-6822(85)90198-9. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]





