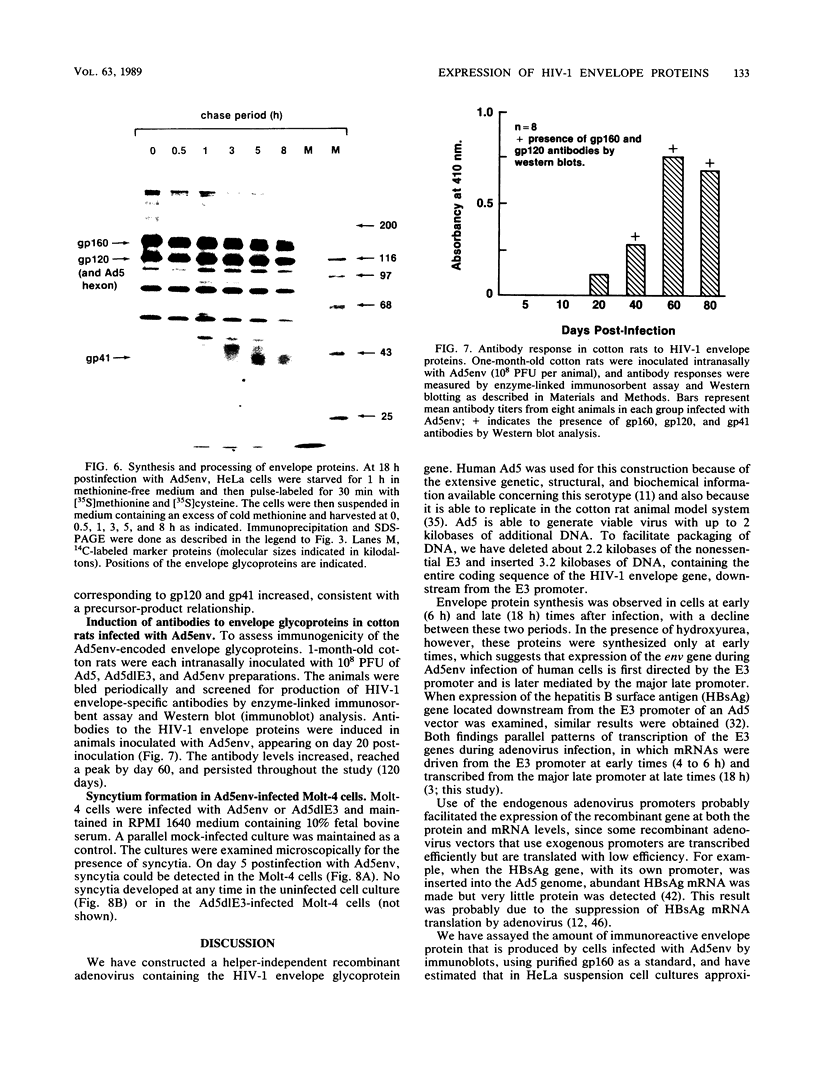
Abstract
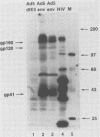
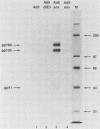
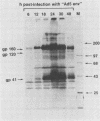
A recombinant adenovirus was constructed by inserting the human immunodeficiency virus type 1 (HIV-1) envelope gene downstream from the early region 3 (E3) promoter of adenovirus type 5 (Ad5), replacing the coding sequences of E3. The recombinant virus replicated as efficiently as the parent virus in all cell lines tested. Human cells infected with the recombinant virus synthesized the HIV-1 envelope precursor gp160, which was efficiently processed to the envelope glycoproteins gp120 and gp41. A human T-lymphoblast line (Molt-4) infected with the recombinant virus expressed HIV-1 envelope glycoproteins on the cell surface, leading to syncytium formation. The envelope gene was expressed from the E3 promoter at early times after infection and at late times from the major late promoter. When cotton rats were infected with the recombinant virus, antibodies against the HIV-1 envelope glycoproteins could be expressed in an immunoreactive form by the recombinant adenovirus, further illustrating the usefulness of adenoviruses as expression vectors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B. M., Wold W. S. Genetic analysis of mRNA synthesis in adenovirus region E3 at different stages of productive infection by RNA-processing mutants. J Virol. 1986 Oct;60(1):54–63. doi: 10.1128/jvi.60.1.54-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Robert-Guroff M., Wong-Staal F., Gallo R. C., Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986 Apr 10;320(6062):535–537. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- Crossland L. D., Raskas H. J. Identification of adenovirus genes that require template replication for expression. J Virol. 1983 Jun;46(3):737–748. doi: 10.1128/jvi.46.3.737-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Ganguly K., Gordon M., Conroy R., Schaber M., Kramer R., Shaw G., Wong-Staal F., Reddy E. P. HTLV-III env gene products synthesized in E. coli are recognized by antibodies present in the sera of AIDS patients. Cell. 1985 Jul;41(3):979–986. doi: 10.1016/s0092-8674(85)80078-7. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Current issues in developing a strategy for dealing with the acquired immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9278–9283. doi: 10.1073/pnas.83.24.9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Sch5AAFER W., Bolognesi D. P. Neutralization of homologous and heterologous oncornaviruses by antisera against the p15(E) and gp71 polypeptides of Friend murine leukemia virus. Virology. 1976 May;71(1):169–184. doi: 10.1016/0042-6822(76)90103-3. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Giorno R., Kates J. R. Mechanism of inhibition of vaccinia virus replication in adenovirus-infected HeLa cells. J Virol. 1971 Feb;7(2):208–213. doi: 10.1128/jvi.7.2.208-213.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Kosowski S. G., Schaaf K. F. Expression of envelope glycoproteins of human immunodeficiency virus by an insect virus vector. J Virol. 1987 Nov;61(11):3617–3620. doi: 10.1128/jvi.61.11.3617-3620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Schäfer W. Properties of mouse leukemia viruses. IX. Active and passive immunization of mice against Friend leukemia with isolated viral GP71 glycoprotein and its corresponding antiserum. Virology. 1975 Jul;66(1):327–329. doi: 10.1016/0042-6822(75)90203-2. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Pedersen N. C., Theilen G. H., Bayer H. Active immunization with feline leukemia virus envelope glycoprotein suppresses growth of virus-induced feline sarcoma. Med Microbiol Immunol. 1983;171(4):233–241. doi: 10.1007/BF02123497. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Schneider J., Schulz A. Immunoprevention of Friend virus-induced erythroleukemia by vaccination with viral envelope glycoprotein complexes. Virology. 1981 Sep;113(2):602–612. doi: 10.1016/0042-6822(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Pedersen N. C., Lerche N. W., Osborn K. G., Lowenstine L. J., Lackner A. A., Maul D. H., Kwang H. S., Kluge J. D., Zaiss C. P. Prevention of simian acquired immune deficiency syndrome with a formalin-inactivated type D retrovirus vaccine. J Virol. 1986 Nov;60(2):431–435. doi: 10.1128/jvi.60.2.431-435.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Robey W. G., Gonda M. A., Carter S. G., Fischinger P. J. Absence of cytotoxic antibody to human immunodeficiency virus-infected cells in humans and its induction in animals after infection or immunization with purified envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3797–3801. doi: 10.1073/pnas.84.11.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Copeland T. D., Simek S., Oroszlan S., Gilden R. V. Detection and characterization of the protein encoded by the v-rel oncogene. Virology. 1986 Mar;149(2):217–229. doi: 10.1016/0042-6822(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Lynn D. L., Robert-Guroff M., Langlois A. J., Lyerly H. K., Carson H., Krohn K., Ranki A., Gallo R. C., Bolognesi D. P. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6924–6928. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Oya Y., Yamamoto K., Yuasa T., Shimojo H. Construction of nondefective adenovirus type 5 bearing a 2.8-kilobase hepatitis B virus DNA near the right end of its genome. J Virol. 1985 Jun;54(3):711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Construction of adenovirus expression vectors by site-directed in vivo recombination. J Mol Appl Genet. 1982;1(5):435–446. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]