Abstract
The fungal pathogen Ustilago hordei causes the covered smut disease of barley and oats. Mating and pathogenicity in this fungus are controlled by the MAT locus, which contains two distinct gene complexes, a and b. In this study, we tagged the a and b regions with the recognition sequence for the restriction enzyme I-SceI and determined that the distance between the complexes is 500 kb in a MAT-1 strain and 430 kb in a MAT-2 strain. Characterization of the organization of the known genes within the a and b gene complexes provided evidence for nonhomology and sequence inversion between MAT-1 and MAT-2. Antibiotic-resistance markers also were used to tag the a gene complex in MAT-1 strains (phleomycin) and the b gene complex in MAT-2 strains (hygromycin). Crosses were performed with these strains and progeny resistant to both antibiotics were recovered at a very low frequency, suggesting that recombination is suppressed within the MAT region. Overall, the chromosome homologues carrying the MAT locus of U. hordei share features with primitive sex chromosomes, with the added twist that the MAT locus also controls pathogenicity.
Keywords: MAT locus, sex chromosome, smut disease, phytopathogen, basidiomycete
Ustilago hordei represents a group of fungal pathogens that cause economically important smut diseases on small-grain cereals (1). These fungi grow as hyphae within developing seedlings without causing symptoms; upon flowering of the host plant, the fungal cells proliferate and form masses of dark teliospores that replace the seeds. The diploid teliospores germinate and undergo meiosis to yield haploid cells. These meiotic progeny must mate to infect the host, and infection is a prerequisite for completion of the sexual phase of the life cycle, i.e., the formation of teliospores. Sex and pathogenicity therefore are interconnected in U. hordei and related smut fungi, and the mating-type genes are considered pathogenicity factors.
U. hordei has a bipolar mating system controlled by one mating-type locus (MAT) with two alleles or alternative specificities, MAT-1 and MAT-2. DNA hybridization experiments with the well-characterized a and b mating-type genes from the related species, Ustilago maydis, revealed that U. hordei possesses similar mating-type functions located at so-called a and b gene complexes within the MAT locus (2, 3). In contrast to U. hordei, U. maydis has a tetrapolar mating system because the a and b loci are on separate chromosomes and therefore segregate independently during meiosis (3, 4). Thus, the tetrapolar and bipolar mating systems are distinguished by differences in the genomic organizations of the a and b genes (3). In U. maydis, two specificities exist for the a locus: a1 and a2 (5, 6). The a locus encodes cell-type specific pheromones (mfa) as well as the pheromone receptors (pra) that recognize pheromones from compatible mating partners. The b locus of U. maydis is multiallelic and contains two divergently transcribed genes, bE (bEast) and bW (bWest) (4, 7–9). The b locus controls pathogenicity and completion of the life cycle through self vs. nonself recognition between bE and bW polypeptides to establish a regulatory factor (8, 9). The homologues of the a and b genes from U. maydis and U. hordei are conserved in structure and function (2, 3, 10–12). In both species, only cells of opposite mating type, that is, having different specificities at both a and b, successfully mate and form colonies with aerial hyphae (fuz+ reaction); these combinations are infectious when inoculated into host plants. Conversely, haploid strains or incompatible partners of the same mating type form yeast-like colonies and are noninfectious.
Previous studies have shown that a and b are physically linked on the largest chromosome of U. hordei and, together, they encode key functions within the MAT locus (3). In this report, we describe the construction and analysis of strains tagged at the a and b gene complexes to determine the size of the MAT locus. Specifically, we show that the MAT locus extends over a large region and that the size and organization of the locus differs between MAT-1 and MAT-2 strains. In addition, we use the tagged strains to further demonstrate that the region between the a and b gene complexes is suppressed for recombination.
Materials and Methods
Strains and Media.
Strains used in this study are listed in Table 1. Fungal strains were grown and mating tests were performed as described (12, 13).
Table 1.
U. hordei strains
| Strain | Genotype* | Source |
|---|---|---|
| U. hordei 549 | a2b1 (Δb2, UhbWE1, hygr, phleor)† | (11) |
| U. hordei 550 | a2b1 (Δb2, UhbWE1, hygr, phleor)† | (11) |
| U. hordei 551 | a1b2 (Δb1, UhbW2, hygr, phleor)† | (11) |
| U. hordei 552 | a1b2 (Δb1, UhbW2, hygr, phleor)† | (11) |
| U. hordei 4857-4 | a1b1 (wild-type strain) | P. Thomas (11) |
| U. hordei 4857-5 | a2b2 (wild-type strain) | P. Thomas (11) |
| U. hordei 364-62/364-86 | a1b1 (Δpan1∷phleor/SceI)‡ | This work |
| U. hordei 364-86dt21 | a1b1 (Δpan1∷phleor/SceI, ΔbWE1:hygr/SceI)§ | This work |
| U. hordei 365-57/365-71 | a2b2 (ΔbW2∷hygr/SceI)¶ | This work |
| U. hordei 365-57dt51 | a2b2 (ΔbW2∷hygr/SceI, Δpan1∷phleor/SceI)∥ | This work |
*phleor, phleomycin resistance; hygr, hygromycin B resistance; SceI, I-SceI recognition sequence.
† In these strains, the resident b genes were inactivated by insertion of a hygromycin B resistance marker; subsequently, functional b gene complexes (pUhbWE1 or pUhbW2) were integrated at ectopic sites (11).
‡These strains were constructed by transformation of strain 4857-4 with pSce-phleo#4 at a1.
§This strain was constructed by transformation of strain 364-86 with pSceI-Hyg#1 at b1.
¶These strains were constructed by transformation of strain 4857-5 with pSceI-Hyg#1 at b2.
∥This strain was constructed by transformation of strain 365-57 with pSce-phleo#4 at a2.
Plasmid Constructions and Gene Complex Tagging.
The four constructs used to tag the a and b gene complexes in U. hordei were based on the two plasmids, pSceI-Hyg#1 and pSce-phleo#4. The hygromycin B resistance cassette (14) and two annealed oligonucleotides containing the I-SceI recognition sequence (5′-TAGGGATAACAGGGTAAT-3′) were cloned into plasmid pGEM3+ (Promega) to construct pSceI-Hyg#1. Plasmid pSce-phleo#4 was constructed by substituting the hygromycin B resistance cassette of pSceI-Hyg#1 with the phleomycin resistance cassette from pUble10 (15). I-SceI is an intron-homing enzyme from Saccharomyces cerevisiae (Boehringer Mannheim; ref. 16).
The a1 gene complex of U. hordei strain 4857–4 and the a2 gene complex of U. hordei strain 365–57 were tagged with the phleomycin-I-SceI cassette from pSce-phleo#4, creating strains 364–62/364–86 and 365–57dt51, respectively (Table 1; Fig. 1). The b1 gene complex of U. hordei strain 364–86 and the b2 gene complex of U. hordei strain 4857–5 were tagged with the hygromycin B-I-SceI cassette of pSceI-Hyg#1, creating strains 364–86dt21 and 365–57/365–71, respectively (Table 1; Fig. 1). Details about DNA constructs can be obtained from the authors. U. hordei electrotransformation and genomic DNA isolation were performed as described (2, 10). Homologous integration of transforming DNA was confirmed by DNA hybridization (data not shown).
Figure 1.
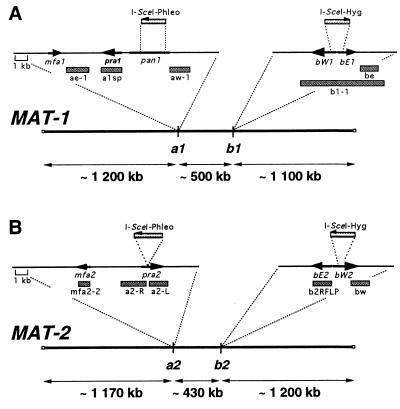
Chromosomal organization of the MAT-1 (A) and MAT-2 (B) loci. The two thick lines represent the MAT chromosomes. The thin lines represent inserts of plasmid constructs used to tag the respective mating-type loci. The mfa, pra, bE, and bW ORFs are shown as black boxed arrows denoting the direction of transcription. The direction of transcription of pan1 and its location in MAT-2 are not known. The locations of probes used for the hybridizations are shown as gray bars. For details on the organization of the gene complexes, see ref. 17.
Pulse-Field Gel Electrophoresis and Hybridization Analysis.
Chromosome-sized DNA from U. hordei was prepared and digested with I-SceI essentially as described (16). Agarose plugs were loaded into 1.2% (wt/vol) agarose gels in 0.5× TBE buffer (45 mM Tris-Borate/1 mM EDTA). Gel electrophoresis was performed at 16°C by using a contour clamped homogenous electric field (CHEF) electrophoresis apparatus (CHEF-DR II, Bio-Rad) under the following conditions: 45-sec pulse at 150 V for 48 hr (Figs. 2 and 3D), 45-sec pulse at 200 V for 48 hr (Fig. 3A), or 3,600-sec pulse ramped to 600-sec pulse at 60 V for 120 hr and 600-sec pulse ramped to 96-sec pulse at 60 V for 50 hr (Fig. 4). The gels were stained with ethidium bromide and treated with 0.25 M HCl, and the DNA was transferred to nylon membranes (Hybond-N+, Amersham Pharmacia). DNA fragments for hybridization probes were selected based on their proximity to the engineered I-SceI sites. For all hybridizations, the probes were labeled with [α-32P]dCTP by random-priming (Amersham Pharmacia). Prehybridizations and hybridizations were in 7% SDS, 0.5 M Na2HPO4 at 65°C, and membranes were washed as described (3).
Figure 2.
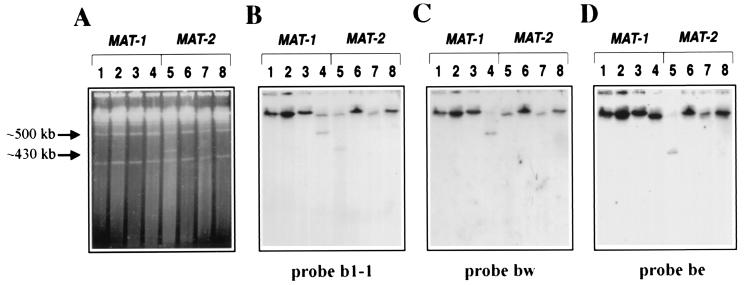
Determination of the size and organization of the MAT locus of U. hordei by hybridization with probes from the b gene complex. (A) Ethidium-bromide-stained CHEF gel. (B–D) DNA gel blots of the same gel hybridized with the probes indicated. Lane 1, I-SceI-digested 4857–4 (a1b1); lane 2, I-SceI-digested 364–86 (a1b1; single tag at a1); lane 3, undigested 364–86dt21 (a1b1; double tag at a1, b1); lane 4, I-SceI-digested 364–86dt21 (a1b1; double tag at a1, b1); lane 5, I-SceI-digested 365–57dt51 (a2b2; double tag at a2, b2); lane 6, undigested 365–57dt51 (a2b2; double tag at a2, b2); lane 7, I-SceI-digested 365–57 (a2b2; single tag at b2); lane 8, I-SceI-digested 4857–5 (a2b2). Note that probes b1–1, be and bw recognize homologous sequences in both MAT loci.
Figure 3.
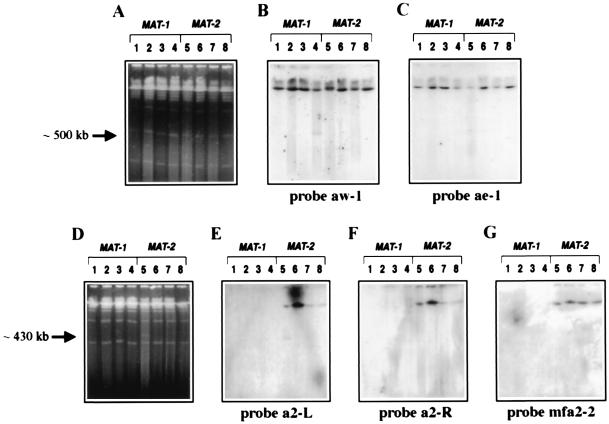
Determination of the size and organization of the MAT locus of U. hordei by hybridization with probes from the a gene complex. (A and D) Ethidium-bromide-stained CHEF gels. (B, C, and E--G) DNA gel blots of the corresponding gels hybridized with the probes as indicated. Lanes are as described in Fig. 2.
Figure 4.
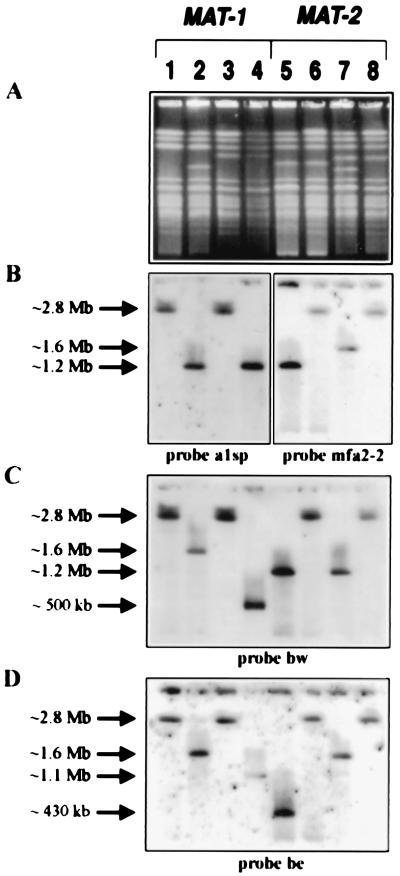
Determination of the chromosomal position of the MAT locus by hybridization with probes from the a and b gene complexes. (A) Ethidium-bromide-stained CHEF gel. (B–D) DNA gel blots of the corresponding gels hybridized with the probes as indicated. Lanes are as described in Fig. 2.
Plant Inoculation and Teliospore Isolation.
Barley seedlings (Hordeum vulgare L.) of cultivars Odessa and 66–2 (gift from P. Thomas, Argiculture and Agri-Food Canada, Winnipeg, MB, Canada) were inoculated with compatible haploid strains of U. hordei as described (11). Teliospores were sterilized with 0.06% sodium hyperchlorite, washed in sterile water, and germinated on Difco potato dextrose agar (PDA). Diploid teliospores were spread on 20 Petri plates to a density of approximately 5,000 teliospores/plate. Metabasidia possessing an average of 12 haploid sporidia were collected from the PDA plates and resuspended in potato dextrose broth (PDB). After vigorous vortexing, cells were plated onto complete medium (CM; ref. 13) to isolate meiotic progeny. For the isolation of double-resistant progeny (drp), cells were plated on CM agar containing both hygromycin B (300 μg/ml, Rose Scientific, Edmonton, Canada) and phleomycin (20 μg/ml, Cayla Laboratories, Toulouse, France). Isolated colonies were picked and resuspended in sterile water. The suspension was vortexed for 5 min and spread onto CM agar containing hygromycin B and phleomycin. Isolated colonies then were grown in PDB for mating tests and for DNA isolation.
Results
The a and b Gene Complexes Are Separated by Large Physical Distances in MAT-1 and MAT-2 Strains.
Initially, we sought to determine the physical distance between the a and b gene complexes within the MAT-1 and MAT-2 loci of U. hordei. We constructed strains that were tagged with the recognition sequence of the rare-cutting restriction enzyme I-SceI at the a1 gene complex (364–86), the b2 gene complex (365–57), or both the a and b gene complexes (364–86dt21 and 365–57dt51; Table 1, Fig. 1). Pulse-field gel electrophoresis and subsequent hybridization analysis of I-SceI-digested chromosome-sized DNA from the double-tagged strains revealed two different-sized fragments representing the regions between a and b for the MAT-1 and MAT-2 strains (Fig. 2). A fragment of ≈500 kb was released upon digestion of DNA from the MAT-1 double-tagged strain. This band comigrated with a 500-kb chromosome and appeared as a doublet in the gel stained with ethidium bromide (Fig. 2A, lane 4). Hybridization with probes from the a and b gene complexes confirmed that this DNA fragment originated from the MAT-1 locus (Figs. 2 B and C, lanes 4, and 3B, lane 4). Similarly, digestion of the double-tagged MAT-2 strain (365–57dt51) with I-SceI released a fragment of ≈430 kb (Fig. 2A, lane 5). This fragment originated from the MAT-2 locus as determined by hybridization with probes from both the a and b gene complexes (Figs. 2 B and D, lane 5, and 3E, lane 5).
The MAT Locus Is in the Center of a 2.8-Mb Chromosome.
The DNA from wild-type, single-tagged, and double-tagged strains was subjected to pulse-field gel electrophoresis for an extended time to position the MAT locus on the chromosome. Interestingly, both MAT-1 and MAT-2 are situated in the central region of an ≈2.8-Mb chromosome (Fig. 1). In MAT-1 strains, probe be hybridized to ≈1.6- and ≈1.1-Mb fragments released upon digestion of DNA from the strain tagged only at the a1 locus and from the strain tagged at both a1 and b1, respectively (Fig. 4D, lanes 2 and 4; see Fig. 1A). As expected, the bw probe hybridized to the same 1.6-Mb fragment released from the single-tagged strain and to the ≈500-kb fragment released from the double-tagged strain (Figs. 4C, lanes 2 and 4, and 2C, lane 4). These data confirmed that the a1 and b1 gene complexes are separated by ≈500 kb (Figs. 2 and 3). The hybridization of an ≈1.2-Mb fragment with probe a1sp showed that the MAT-1 locus is centrally located, with ≈1.2 and ≈1.1 Mb flanking the a1 and b1 gene complexes, respectively (Figs. 4B, lanes 2 and 4, and 1A). Similar results were obtained for MAT-2 strains. For example, probe be detected the 1.6-Mb fragment from the single-tagged strain and the 430-kb fragment from the double-tagged strain (Figs. 4D, lanes 5 and 7, and 1B). Furthermore, both probes mfa2–2 and bw hybridized to ≈1.2-Mb fragments released from the strain tagged at both a2 and b2, revealing that the MAT-2 locus also is located in the middle of an ≈2.8-Mb chromosome (Fig. 4 B and C, lane 5).
The 430- and 500-kb fragments were not detected by hybridization of any probe to the DNA of wild-type strains digested with I-SceI, digested DNA from the single-tagged strains and undigested DNA from the double-tagged strains (Figs. 2 B–D and 3 B, C, E, and F, lanes 1–3 and 6–8). In fact, for all hybridizations, all of the probes hybridized to high molecular weight DNA shown to be the ≈2.8-Mb chromosome from wild-type and undigested double-tagged strains only (Figs. 2–4). We conclude that the 430-and 500-kb DNA fragments were indeed the regions between the a and b gene complexes.
The a and b Gene Complexes Have Different Organizations Within MAT-1 and MAT-2.
Hybridization with probes from either side of the I-SceI site inserted at b revealed that the sequences at this gene complex in the MAT-1 strain were inverted compared with the homologous sequences in the MAT-2 strain. Both the bw and be probes contain homologous DNA sequences from the b1 and b2 gene complexes (Fig. 1; ref. 2). The bw probe hybridized to the 500-kb fragment that was released upon I-SceI digestion of DNA from the double-tagged MAT-1 strain, whereas probe be hybridized to the 430-kb fragment released upon digestion from the double-tagged MAT-2 strain (Figs. 1 and 2 C, lane 4 and D, lane 5). These results indicate that the bW gene is closer to the a locus than the bE gene in MAT-1 strains, and the orientation of bE and bW is reversed in MAT-2 strains (Fig. 1).
Hybridization probes from both sides of the I-SceI site at a1 and a2 were used to explore the overall organization of the a and b gene complexes within MAT-1 and MAT-2. The aw-1 probe hybridized to the 500-kb region between a1 and b1, indicating that the direction of transcription of pra1 is oriented away from the b1 gene complex (Figs. 1A and 3B, lane 4; ref. 17). In support of this conclusion, probe ae-1 hybridized to a higher molecular weight fragment shown to be ≈1.2-Mb (Figs. 1A and 3C, lane 4; comparable with probe a1sp in Fig. 4B, lanes 2 and 4). For MAT-2, the fragment from the 3′ terminus of pra2 (probe a2-L, Fig. 1B) hybridized to the 430-kb fragment released after digestion with I-SceI (Fig. 3E, lane 5). Accordingly, the probe from the 5′ end of pra2 (probe a2-R, Fig. 1B) hybridized only to the higher molecular weight fragment shown to be ≈1.2 Mb (Fig. 3F, lane 5; comparable with probe mfa2–2 in Fig. 4B, lane 5). In addition, the hybridization with probe mfa2–2 (Fig. 1B) showed that mfa2 was located upstream of pra2 and outside of the 430-kb region spanning pra2 and the b gene complex (Fig. 3G, lane 5). The organization of the pra and mfa genes at both a1 and a2 also has been confirmed by PCR and sequence analysis (17). Overall, our results show that the organization of the genes at a1 differs from that of a2; i.e., pra1 and mfa1 are convergently transcribed and pra2 and mfa2 are divergently transcribed.
Interestingly, the probes from the flanking sequences of the I-SceI site at the a2 gene complex (a2-L, a2-R, and mfa2–2; Fig. 1B) did not hybridize to DNA from MAT-1 strains (Figs. 3 E and F, lanes 1–4, and 4B). Probe a1sp, containing the pra1 gene, was also specific for MAT-1 strains (Figs. 4B and 5). These results indicate that regions of nonhomology may exist between the a gene complexes in MAT-1 and MAT-2.
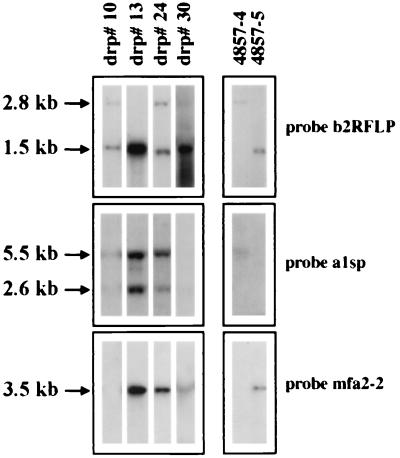
Figure 5.
Identification of mating-type-specific sequences in the drp by hybridization with probes from the a and b gene complexes. DNA gel blots of BamHI-digested genomic DNA from representative drp (see text) and both parental strains were hybridized with the probes indicated.
Recombination Is Suppressed in the Region Between a and b.
In previous work, a screen of 2,000 progeny failed to identify strains with altered mating types caused by recombination within the MAT locus (3). To further investigate the apparent low frequency of recombination in the region between a and b, the gene complexes were tagged with genes for resistance to the antibiotics phleomycin and hygromycin B. Specifically, two strains were constructed by insertion of the phleomycin gene at the a1 locus (364–62 and 364–86) and two strains were obtained with the hygromycin B gene at the b2 locus (365–57 and 365–71) (Fig. 1, Table 1).
The a1-tagged strains each were crossed with the two strains tagged at b2 by coinoculation of barley seedlings. Our goal was to estimate the frequency of recombination between the a and b gene complexes by germinating the teliospores from the crosses and selecting double-resistant recombinant progeny on a medium with both antibiotics. Presumably, only progeny having the recombinant genotype a1b2 (phleor, hygr) would be able to form colonies, and their frequency of appearance would provide a measure of recombination.
Initially, 50 meiotic progeny were isolated from each of the four crosses. The mating type specificities of these strains were determined by plate-mating assays with two wild-type strains, 4857–4 (a1b1) and 4857–5 (a2b2). These progeny also were tested for resistance to phleomycin and hygromycin B. As shown in Table 2, mating type and antibiotic resistance segregated in an approximately 1:1 fashion in three of the four crosses; the fourth cross showed a reduced recovery of MAT-2, phleos, hygr progeny for unknown reasons. As expected, the progeny demonstrated complete linkage between phleomycin resistance and MAT-1 mating specificity and between hygromycin B resistance and MAT-2. Overall, the analysis of the 200 progeny from these crosses indicated that meiotic segregation occurred normally in the strains carrying tagged a1 and b2 sequences.
Table 2.
Segregation of markers in crosses to detect recombination within MAT
| Cross | Ratio of progeny with the following genotypes:
|
|||
|---|---|---|---|---|
| MAT-1/ MAT-2* | phleor/ phleos† | hygs/ hygr† | phleor, hygr/ phleos, hygs‡ | |
| 1 | 24/25 | 24/25 | 24/25 | 6/18,400 |
| 2 | 23/26 | 23/26 | 23/26 | 7/18,400 |
| 3 | 26/24 | 26/24 | 26/24 | 10/17,800 |
| 4 | 35/15 | 35/15 | 35/15 | 11/17,400 |
| Total | 108/90 | 108/90 | 108/90 | 34/72,000 |
The genotypes of the progeny were determined by performing mating tests with wild-type strains 4857-4 and 4857-5.
† Two hundred random progeny were tested for resistance to phleomycin (phleor) or hygromycin B (hygr).
‡ For each cross, 20, 000 progeny were inoculated onto culture medium. An average viability of ∼90% was obtained for each cross. A total of 72,000 viable progeny were tested for resistance to both antibiotics. A second trial identified 36 progeny (out of 70,420) that were resistant to both antibiotics.
To determine the frequency of recombination between the a and b gene complexes, a total of 72,000 isolates from the four crosses were plated on a medium containing both antibiotics. Considering the number of germinated spores used and the number of progeny per spore (average of 12), we estimated that our sample represented between 10,000 and 20,000 random progeny. The selection for drp yielded only 34 colonies, suggesting that recombination was indeed greatly suppressed within the MAT region despite the 400- to 500-kb distance separating a and b. The 34 drp subsequently were tested for their mating specificity in assays with two wild-type strains (4857–4 and 4857–5) and four strains engineered with artificial combinations of a and b (549, 550, 551, and 552; Table 1; ref. 11). Interestingly, none of the drp mated solely with the 549 or 550 tester strains, and the majority displayed unusual mating behaviors. Although numerous independent mating tests were performed, most of the progeny either failed to consistently give a positive mating reaction with any tester strain or mated routinely with more than one tester (data not shown).
The possibility that the drp contained both selectable markers because of the maintenance of both parental copies of the MAT chromosome rather than recombination was explored by a molecular test for the presence of the a and b gene complexes from both MAT-1 and MAT-2. Representative hybridization results are shown in Fig. 5. Specifically, the b2RFLP hybridization probe was used to detect a restriction fragment length polymorphism (RFLP) that segregates with mating type (Figs. 1B and 5; ref. 10). Hybridization of this probe to DNA from the progeny revealed that many (13/34) contained both RFLP fragments, indicating the presence of both b1 and b2. Interestingly, hybridization with a1- and a2-specific probes revealed that most of the drp (32/34) contained both a1- and a2-specific sequences (Fig. 5). In addition, all of the drp carrying a1-specific sequences showed an unusual hybridization pattern in that both a wild-type fragment of 5.5 kb and a unique 2.6-kb fragment hybridized to probe a1sp (Fig. 5). Possibly, the extra fragment arose from a recombination event that resulted in a duplication of part of the pra1 gene. Overall, the molecular markers demonstrated in Fig. 5 were found in the following combinations in the 34 progeny: a1, b1, b2 (1/34; e.g., drp#10); mfa2, b1, b2 (1/34; e.g., drp#30); a1, mfa2, b1, b2 (11/34; e.g., drp#24); a1, mfa2, b2 (21/34; e.g., drp#13). In summary, these results indicated that the double-resistant phenotype was not the result of a simple reciprocal recombination event between a and b in the 34 drp analyzed. Rather, the drp most likely arose from other events such as rearrangements at a or b or the retention of all or part of both MAT chromosome homologues to yield aneuploid, or perhaps, diploid strains. It also should be noted that true reciprocal recombinant progeny from our experiments may have been inviable and therefore not detected, although a1b2 and a2b1 strains have been constructed (11).
Discussion
The MAT Locus of U. hordei Is Larger Than Previously Characterized Fungal Mating Type Loci.
Our experiments indicate that the MAT locus for U. hordei is surprisingly large compared with the mating-type sequences characterized in other fungi (18). Among fungi, the largest mating-type regions have been described in basidiomycetes. For example, the mushroom Coprinus cinereus possesses mating-type loci of approximately 30 kb (A42 locus) and 17 kb (B6 locus) (19, 20). The A and B loci of C. cinereus encode proteins with functional similarity to the b- and a-encoded proteins of U. hordei, respectively. The MAT locus of the human pathogen Cryptococcus neoformans is 35–45 kb in size, although it has been suggested recently that the locus may be as large as 75 kb (21, 22). This locus has been implicated in virulence (23).
In the context of pathogenicity, the large size and multigenic nature of the MAT locus of U. hordei is reminiscent of complex pathogenicity regions that segregate as one locus in other phytopathogenic fungi. For example, the Tox2 locus of Cochliobolus carbonum is responsible for the production of a host-specific toxin, which promotes lesion formation on leaves of susceptible host plants. The genetic functions for toxin synthesis segregate as a single unit, although Tox2 spans a region of >500 kb (24). In Nectria haematococca, the genes required for pathogenicity to pea (PEP) have been localized to a dispensable chromosome that is proposed to be suppressed for recombination (25). Furthermore, genes involved in the biosynthesis of elicitins are clustered in Phytophthora cryptogea, as are mycotoxin biosynthetic genes in Fusarium species, Aspergillus nidulans, and Gibberella fujikuroi (26–29). These examples make it tempting to speculate that other genes involved in mating and pathogenesis might be clustered along with the a and b genes at the MAT locus of U. hordei. In other words, recombination suppression may serve to maintain a set of genes together that function in the sexual development of the fungus in the host. Further characterization of the sequences between a and b will elucidate the organization of this region, show the relationship between MAT-1 and MAT-2, and, perhaps, reveal additional genes involved in mating and pathogenesis.
Recombination on the MAT Chromosome of U. hordei.
Although recombination was investigated only in the region between the a and b gene complexes in this study, it is conceivable that regions outside of these gene complexes may be suppressed for recombination as well. Precedent exists for suppression extending over large distances in fungi, e.g., the absence of recombination between several loci spanning almost the entire chromosome carrying the mating-type locus has been reported for Neurospora tetrasperma (30). Very few genetic markers have been identified in U. hordei, and it is therefore difficult to assess the relationship between physical and genetic distance over any portion of the genome. A limited number of studies have explored recombination between the MAT locus and linked markers, and these indicate that crossing over occurs on the MAT chromosome. For example, Groth (31) reported a recombination frequency of 10.8% between MAT and a gene conditioning a mycelial phenotype for haploid cells. In addition, Henry et al. (32) reported a recombination frequency of 12% for the pan-1 and pro-2 genes in U. hordei; the pan-1 gene is known to be located within or near the a gene complex in both U. hordei (Fig. 1A) and U. maydis (5). Thomas (33) examined recombination between the MAT locus, pan1, and pro1 among 361 ordered tetrads of U. hordei. All but eight tetrads had the parental combination of markers indicating tight linkage of all three traits. The remaining eight tetrads showed unusual segregation for one or more of the markers (e.g., 3:1 and 4:0 ratios) suggesting that gene conversion events may have occurred. In fact, two of these tetrads segregated 3:1 for MAT. In general, further characterization of the MAT locus will require additional physical and genetic mapping to generate a more detailed picture of recombination frequencies across the chromosome and to clarify the actual size of the MAT locus.
Does U. hordei Possess Primitive Dimorphic Sex Chromosomes?
The features of the MAT locus such as recombination suppression, insertion/deletions, inversion, regions of nonhomology between MAT-1 and MAT-2, and the presence of sex-determining genes, are reminiscent of the X/Y sex-chromosome systems (34–36). A striking similarity between the mating system of the lower eukaryote Chlamydomonas reinhardtii and sex chromosomes in higher eukaryotes already has been described (36, 37). Intrachromosomal translocations, inversions, duplications, and large deletions are associated with the mt locus and are thought to contribute to the suppression of recombination within a 830-kb stretch of DNA (37). Suppression of recombination at mating-type loci has been attributed to a variety of mechanisms including nonhomology of genes at the locus, different complements of genes, chromosomal rearrangements, or a special chromatin structure (18, 37). The MAT locus of U. hordei appears to share features with the mt locus of C. reinhardtii that may contribute to suppressed recombination, although a more detailed characterization of the U. hordei locus is needed. In particular, it will be of interest to search the MAT locus for additional features that may shed light on the evolution of sex chromosomes, such as repetitive sequences and transposable elements (36, 37). This search also will enhance our understanding of the mechanism by which recombination is suppressed between MAT-1 and MAT-2; it may be the case, for example, that recombination suppression within the centrally located MAT locus may be caused by the presence of the centromere. Further analysis of the chromosomes carrying MAT in U. hordei may contribute to an understanding of the evolution of dimorphic sex chromosomes and mating-type loci in fungi.
Acknowledgments
We thank Dr. Percy Thomas for U. hordei strains and Deborah Willits for technical assistance. This work was supported by the Natural Sciences and Engineering Research Council of Canada (J.W.K.) and the U.S. Department of Agriculture/National Research Initiative (#94–37303–0765; J.E.S.).
Abbreviations
- MAT
mating-type locus
- CHEF
clamped homogenous electric field
- drp
double-resistant progeny
References
- 1.Thomas P L. Adv Plant Pathol. 1988;6:415–425. [Google Scholar]
- 2.Bakkeren G, Kronstad J W. Plant Cell. 1993;5:123–136. doi: 10.1105/tpc.5.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakkeren G, Kronstad J W. Proc Natl Acad Sci USA. 1994;91:7085–7089. doi: 10.1073/pnas.91.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowell J B, DeVay J E. Phytopathology. 1954;44:356–362. [Google Scholar]
- 5.Froeliger E H, Leong S A. Gene. 1991;100:113–122. doi: 10.1016/0378-1119(91)90356-g. [DOI] [PubMed] [Google Scholar]
- 6.Bölker M, Urban M, Kahmann R. Cell. 1992;68:441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- 7.Puhalla J E. Genetics. 1968;60:461–474. doi: 10.1093/genetics/60.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillissen B, Bergemann J, Sandmann C, Schroeer B, Bölker M, Kahmann R. Cell. 1992;68:647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- 9.Kämper J, Reichmann M, Romeis T, Bölker M, Kahmann R. Cell. 1995;81:73–83. doi: 10.1016/0092-8674(95)90372-0. [DOI] [PubMed] [Google Scholar]
- 10.Bakkeren G, Gibbard B, Yee A, Froeliger E H, Leong S A, Kronstad J W. Mol Plant-Microbe Interact. 1992;5:347–355. doi: 10.1094/mpmi-5-347. [DOI] [PubMed] [Google Scholar]
- 11.Bakkeren G, Kronstad J W. Genetics. 1996;143:1601–1613. doi: 10.1093/genetics/143.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Espinoza A D, Gerhardt S A, Sherwood J E. Exp Mycol. 1993;17:200–214. [Google Scholar]
- 13.Holliday R. In: Handbook of Genetics. King R C, editor. Vol. 1. New York: Plenum; 1974. pp. 575–595. [Google Scholar]
- 14.Kronstad J W, Leong S A. Genes Dev. 1990;4:1384–1395. doi: 10.1101/gad.4.8.1384. [DOI] [PubMed] [Google Scholar]
- 15.Gold S, Bakkeren G, Davies J E, Kronstad J W. Gene. 1994;142:225–230. doi: 10.1016/0378-1119(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 16.Thierry A, Dujon B. Nucleic Acids Res. 1992;20:5625–5631. doi: 10.1093/nar/20.21.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson C M, Willits D A, Kosted P J, Ford E, Martinez-Espinoza A D, Sherwood J E. Gene. 1999;240:89–97. doi: 10.1016/s0378-1119(99)00428-x. [DOI] [PubMed] [Google Scholar]
- 18.Kronstad J W, Staben C. Annu Rev Genet. 1997;31:245–267. doi: 10.1146/annurev.genet.31.1.245. [DOI] [PubMed] [Google Scholar]
- 19.Kües U, Asante-Owusu R N, Mutasa E S, Tymon A M, Pardo E H, O'Shea S F, Göttgens B, Casselton L A. Plant Cell. 1994;6:1467–1475. doi: 10.1105/tpc.6.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Shea S F, Chaure P T, Halsall J R, Olesnicky N S, Laibbrandt A, Connerton I F, Casselton L A. Genetics. 1998;148:1081–1090. doi: 10.1093/genetics/148.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore T D E, Edman J C. Mol Cell Biol. 1993;13:1962–1970. doi: 10.1128/mcb.13.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickes B L, Edman U, Edman J C. Mol Microbiol. 1997;26:951–960. doi: 10.1046/j.1365-2958.1997.6322001.x. [DOI] [PubMed] [Google Scholar]
- 23.Kwon-Chung K J, Edman J C, Wickes B L. Infect Immun. 1992;60:602–605. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn J-H, Walton J. Plant Cell. 1996;8:887–897. doi: 10.1105/tpc.8.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanEtten H, Wasmann C, McCluskey K. In: Advances in Molecular Genetics of Plant-Microbe Interactions. Daniels M J, Downie J A, Osbourn A E, editors. Vol. 3. Dordrecht, The Netherlands: Kluwer; 1994. pp. 163–170. [Google Scholar]
- 26.Panabières F, Marais A, Le Berre J-Y, Penot I, Fournier D, Ricci P. Mol Plant-Microbe Interact. 1995;8:996–1003. doi: 10.1094/mpmi-8-0996. [DOI] [PubMed] [Google Scholar]
- 27.Desjardins A E, Hohn T M, McCormick S P. Microbiol Rev. 1993;57:595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desjardins A E, Plattner R D, Proctor R H. Appl Environ Microbiol. 1996;62:2571–2576. doi: 10.1128/aem.62.7.2571-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown D W, Yu J-H, Kelkar H S, Fernandes M, Nesbitt T C, Keller N P, Adams T H, Leonard T J. Proc Natl Acad Sci USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merino S T, Nelson M A, Jacobson D J, Natvig D O. Genetics. 1996;143:789–799. doi: 10.1093/genetics/143.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groth J V. Can J Bot. 1975;53:2233–2239. [Google Scholar]
- 32.Henry C E, Bullock B, Smith V, Steward-Clark E. Bot Gaz. 1988;149:101–106. [Google Scholar]
- 33.Thomas P L. Annu Rev Phytopathol. 1991;29:137–148. [Google Scholar]
- 34.Jablonka E, Lamb M J. Biol Rev Camb. 1990;65:249–276. doi: 10.1111/j.1469-185x.1990.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 35.Charlesworth B. Science. 1991;251:1030–1033. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 36.Charlesworth B. Curr Biol. 1994;4:739–741. doi: 10.1016/s0960-9822(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 37.Ferris P J, Goodenough U W. Cell. 1994;76:1135–1145. doi: 10.1016/0092-8674(94)90389-1. [DOI] [PubMed] [Google Scholar]