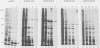Abstract
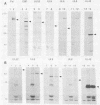
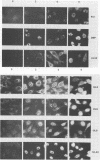
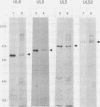
Seven herpes simplex virus (HSV) genes have been shown recently to be necessary and sufficient to support the replication of origin-containing plasmids. Two of these genes (pol and dbp) encode well-known DNA replication proteins (the DNA polymerase and the major single-stranded DNA binding protein), and a third gene (UL42) encodes a previously identified infected-cell protein which binds tightly to double-stranded DNA. The products of the four remaining genes have not previously been identified. Using the predicted amino acid sequence data (D.J. McGeoch, M.A. Dalrymple, A. Dolan, D. McNab, L.J. Perry, P. Taylor, and M.D. Challberg, J. Virol. 62:444-453; D.J. McGeoch and J.P. Quinn, Nucleic Acids Res. 13:8143-8163), we have raised rabbit antisera against the products of all seven genes. We report here the use of these reagents to identify these proteins in infected cells. All seven proteins localized to the nucleus and were expressed in a manner consistent with the idea that they are the products of early genes. Various immunological assays suggest that four of these proteins (UL5, UL8, UL9, and UL52) are made in infected cells in very low abundance relative to the other three. To improve our ability to study these proteins, we have expressed UL5, UL8, UL9, and UL52 in insect cells by using the baculovirus expression system. The HSV protein made in insect cells were immunoprecipitable with the appropriate antisera, and the size of each protein was indistinguishable from the size of the corresponding protein made in HSV-infected Vero cells. Our data offer strong support for the accuracy of open reading frames proposed by McGeoch et al. In addition, the antisera and the overproduced HSV replication proteins should be useful reagents with which to analyze the biochemistry of HSV DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L., Purifoy D. J., Hurst P. F., Killington R. A., Powell K. L. Herpes simplex virus non-structural proteins. IV. Purification of the virus-induced deoxyribonuclease and characterization of the enzyme using monoclonal antibodies. J Gen Virol. 1983 Oct;64(Pt 10):2249–2260. doi: 10.1099/0022-1317-64-10-2249. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Carmichael E. P., Kosovsky M. J., Weller S. K. Isolation and characterization of herpes simplex virus type 1 host range mutants defective in viral DNA synthesis. J Virol. 1988 Jan;62(1):91–99. doi: 10.1128/jvi.62.1.91-99.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., O'Donnell M. E., Mocarski E. S., Lehman I. R. A DNA binding protein specific for an origin of replication of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6322–6326. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988 Aug;62(8):2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. M., Wietstock S. M., Ruyechan W. T. Identification and characterization of a DNA primase activity present in herpes simplex virus type 1-infected HeLa cells. J Virol. 1988 Mar;62(3):1038–1045. doi: 10.1128/jvi.62.3.1038-1045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchetti M. E., Smith C. A., Schaffer P. A. A temperature-sensitive mutation in a herpes simplex virus type 1 gene required for viral DNA synthesis maps to coordinates 0.609 through 0.614 in UL. J Virol. 1988 Mar;62(3):715–721. doi: 10.1128/jvi.62.3.715-721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Campbell M. E., Haarr L., Frame M. C., Parris D. S., Murphy M., Hope R. G., Muller M. T., Preston C. M. The 65,000-Mr DNA-binding and virion trans-inducing proteins of herpes simplex virus type 1. J Virol. 1987 Aug;61(8):2428–2437. doi: 10.1128/jvi.61.8.2428-2437.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz B., Subak-Sharpe J. H., Preston V. G. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 using cloned restriction endonuclease fragments. J Gen Virol. 1983 Oct;64(Pt 10):2261–2270. doi: 10.1099/0022-1317-64-10-2261. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Herpesvirus-dependent amplification and inversion of cell-associated viral thymidine kinase gene flanked by viral a sequences and linked to an origin of viral DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5626–5630. doi: 10.1073/pnas.79.18.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus DNA replication: the UL9 gene encodes an origin-binding protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5414–5418. doi: 10.1073/pnas.85.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Cross A., Haarr L., Orr A., Frame M. C., Murphy M., McGeoch D. J., Marsden H. S. Identification of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J Virol. 1988 Mar;62(3):818–825. doi: 10.1128/jvi.62.3.818-825.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Palfreyman J. W., Dutia B. M. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J Gen Virol. 1984 Sep;65(Pt 9):1457–1466. doi: 10.1099/0022-1317-65-9-1457. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Fraser M. J., Summers M. D. Molecular Engineering of the Autographa californica Nuclear Polyhedrosis Virus Genome: Deletion Mutations Within the Polyhedrin Gene. J Virol. 1983 May;46(2):584–593. doi: 10.1128/jvi.46.2.584-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Localization of an origin of DNA replication within the TRS/IRS repeated region of the herpes simplex virus type 1 genome. EMBO J. 1982;1(7):863–867. doi: 10.1002/j.1460-2075.1982.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Purifoy D. J., Powell K. L. DNA-binding protein associated with herpes simplex virus DNA polymerase. J Virol. 1985 Feb;53(2):501–508. doi: 10.1128/jvi.53.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlazny D. A., Frenkel N. Replication of herpes simplex virus DNA: localization of replication recognition signals within defective virus genomes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):742–746. doi: 10.1073/pnas.78.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Use of a bacterial expression vector to identify the gene encoding a major core protein of vaccinia virus. J Virol. 1985 Nov;56(2):534–540. doi: 10.1128/jvi.56.2.534-540.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]