Abstract
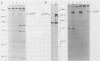
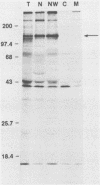
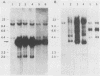
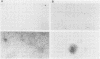
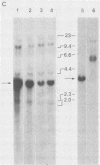
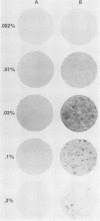
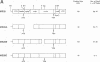
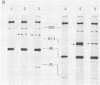
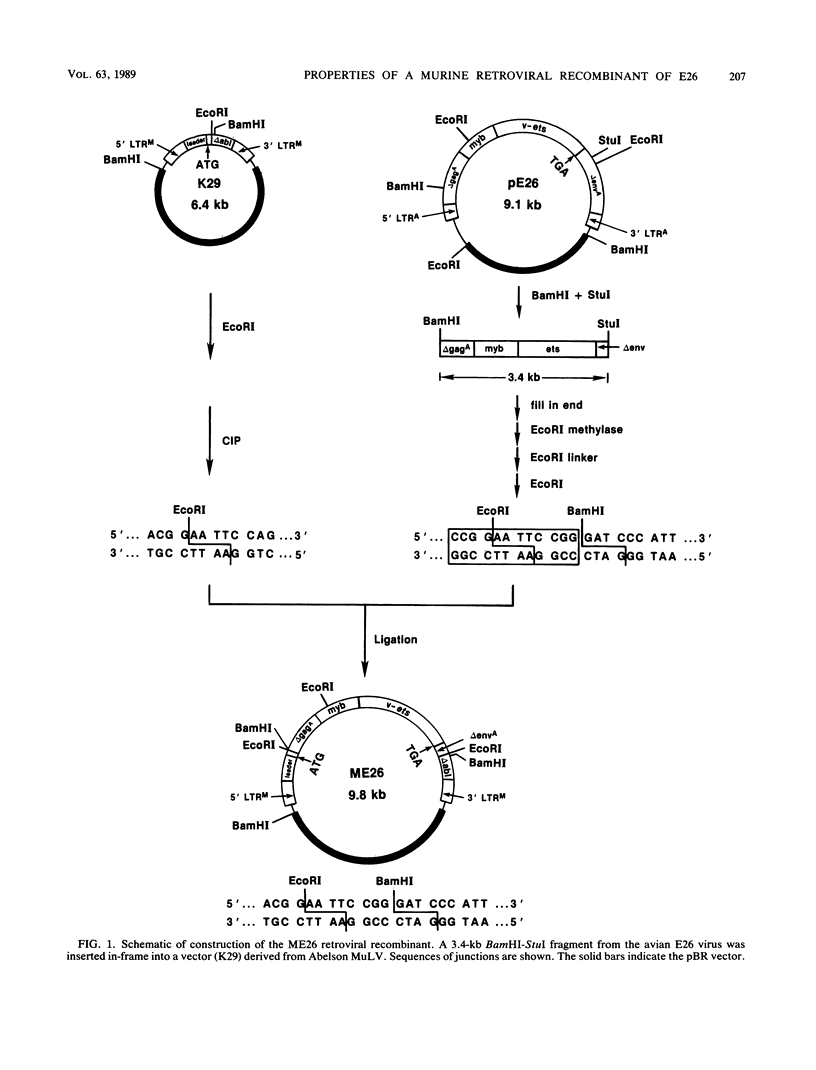
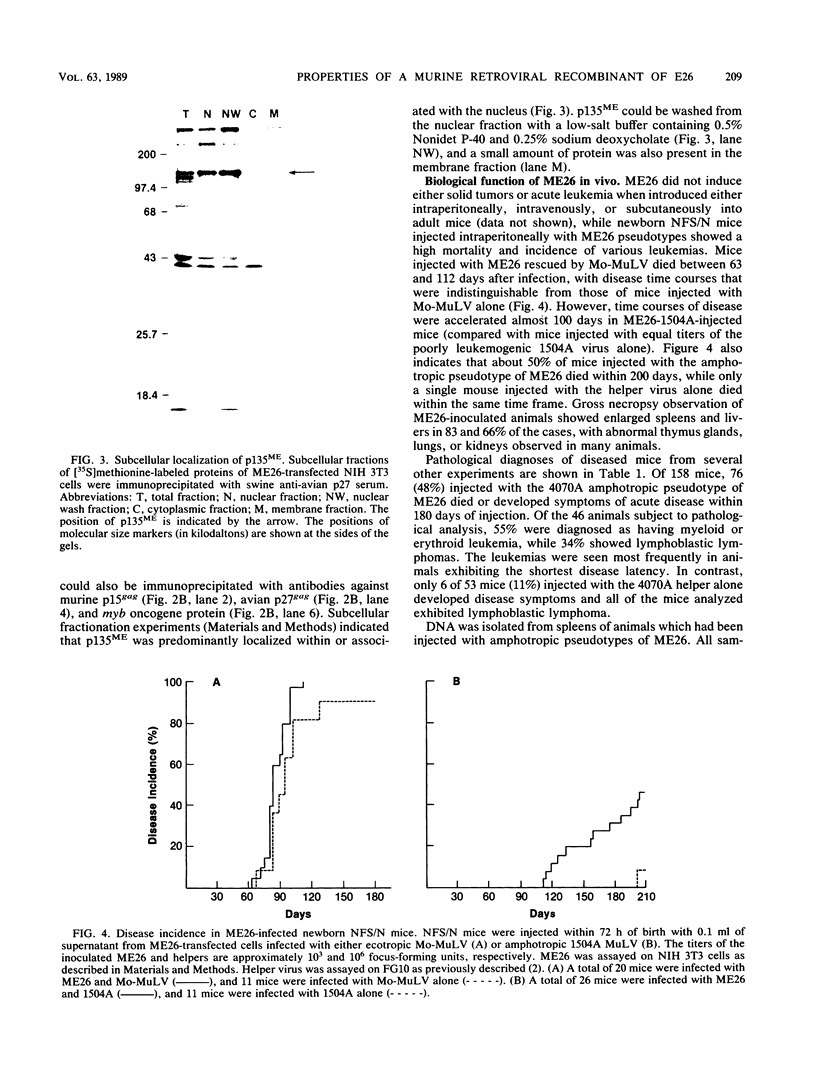
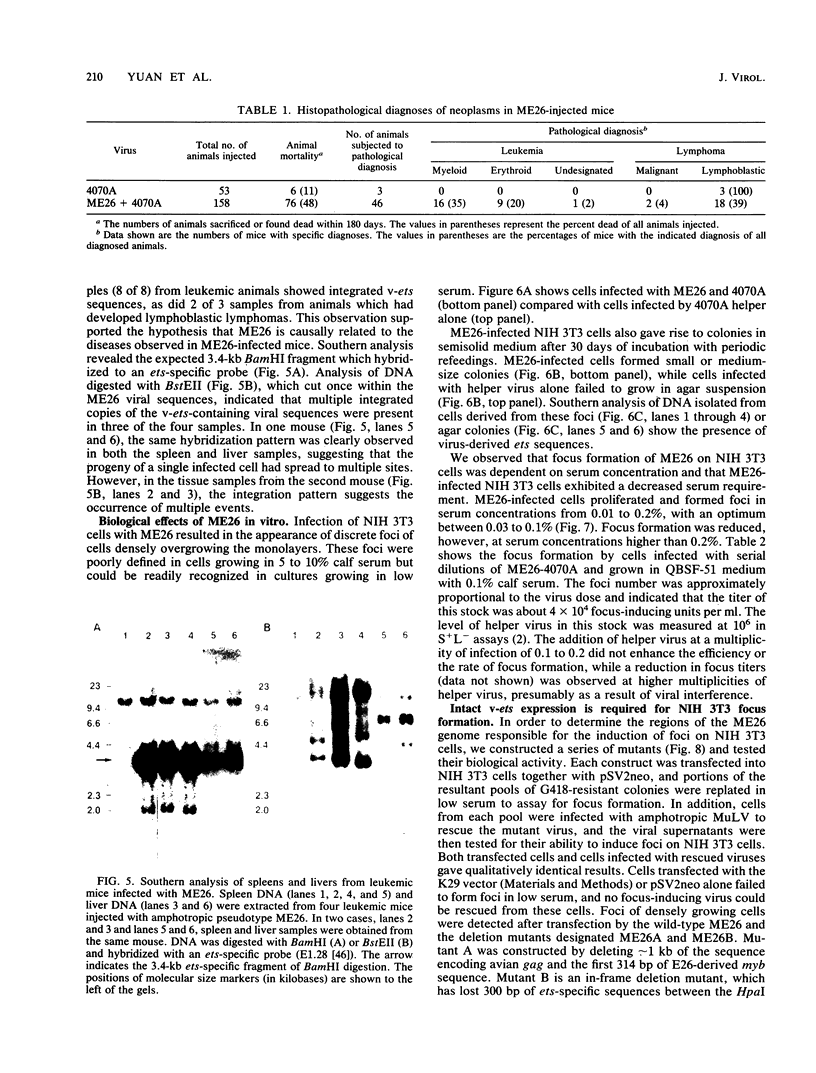
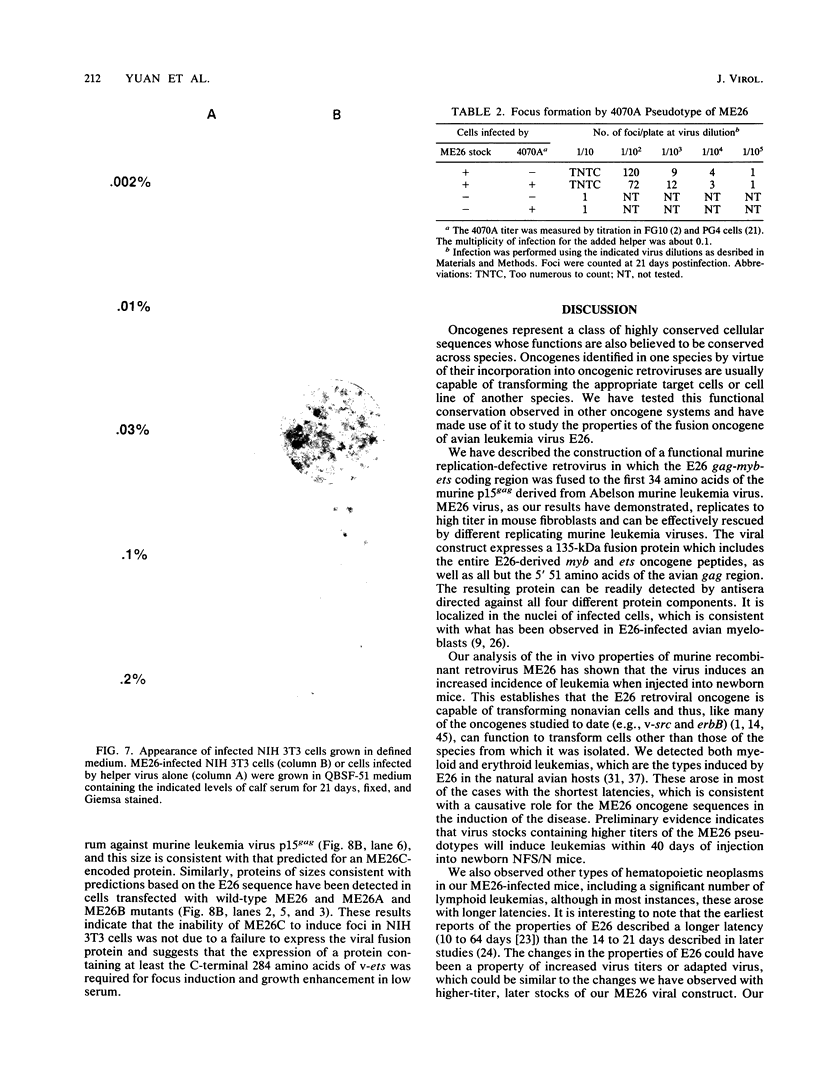
A replication-defective murine retroviral construct, termed pME26, was generated by inserting avian gag-myb-ets sequences derived from the cloned avian acute leukemia virus E26 into an Abelson murine leukemia virus-derived retroviral vector. ME26 virus can be rescued efficiently from transfected NIH 3T3 cells by replicating murine leukemia viruses. Either pME26-transfected nonproducers or ME26 virus-infected NIH 3T3 cells expressed a 135-kilodalton fusion protein (p135) which was detectable by immunoprecipitation with antiserum directed against avian leukemia virus p27gag, myb or ets oncogene protein, or murine leukemia virus p15gag and was principally localized in the nucleus. NIH 3T3 cells infected with ME26 exhibited morphological alterations and increased proliferation in reduced serum and formed small colonies in agar suspension. Discrete foci could be readily recognized in cells maintained in a defined medium containing 0.03 to 0.1% calf serum. In newborn NFS/N mice, ME26 induced a significantly higher mortality and incidence of erythroid and myeloid leukemias. Analysis of a series of mutants affecting the expression of various portions of p135 indicated that the v-ets gene acts to mitogenically stimulate the proliferation of NIH 3T3 fibroblasts and reduces or abolishes their serum dependence. These properties provide an assay system to study functions of the ets gene family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Leutz A., Kahn P., Graf T. Ts mutants of E26 leukemia virus allow transformed myeloblasts, but not erythroblasts or fibroblasts, to differentiate at the nonpermissive temperature. Cell. 1984 Dec;39(3 Pt 2):579–588. doi: 10.1016/0092-8674(84)90465-3. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Begue A., Galibert F., Gesquière J. C., Stéhelin D., Ghysdael J. Identification in chickens of an evolutionarily conserved cellular ets-2 gene (c-ets-2) encoding nuclear proteins related to the products of the c-ets proto-oncogene. EMBO J. 1988 Mar;7(3):697–705. doi: 10.1002/j.1460-2075.1988.tb02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Lipsick J. S., Baluda M. A. Avian myeloblastosis virus and E26 virus oncogene products are nuclear proteins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4265–4269. doi: 10.1073/pnas.81.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Q., Kan N. C., Pribyl L., Lautenberger J. A., Moudrianakis E., Papas T. S. Molecular cloning of the ets proto-oncogene of the sea urchin and analysis of its developmental expression. Dev Biol. 1988 Feb;125(2):432–440. doi: 10.1016/0012-1606(88)90224-2. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Le Beau M. M., Pitha P., Rowley J. D. Interferon and c-ets-1 genes in the translocation (9;11)(p22;q23) in human acute monocytic leukemia. Science. 1986 Jan 17;231(4735):265–267. doi: 10.1126/science.3455787. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Bishop J. M. Isolation of monoclonal antibodies specific for products of avian oncogene myb. Mol Cell Biol. 1984 Dec;4(12):2843–2850. doi: 10.1128/mcb.4.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Pierce J. H., Kraus M. H., Di Fiore P. P., Pennington C. Y., Aaronson S. A. Mammalian cell transformation by a murine retrovirus vector containing the avian erythroblastosis virus erbB gene. J Virol. 1986 Oct;60(1):19–28. doi: 10.1128/jvi.60.1.19-28.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegonne A., Leprince D., Duterque-Coquillaud M., Vandenbunder B., Flourens A., Ghysdael J., Debuire B., Stehelin D. Multiple domains for the chicken cellular sequences homologous to the v-ets oncogene of the E26 retrovirus. Mol Cell Biol. 1987 Feb;7(2):806–812. doi: 10.1128/mcb.7.2.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegonne A., Leprince D., Pognonec P., Dernis D., Raes M. B., Stehelin D., Ghysdael J. The 5' extremity of the v-ets oncogene of avian leukemia virus E26 encodes amino acid sequences not derived from the major c-ets-encoded cellular proteins. Virology. 1987 Jan;156(1):177–180. doi: 10.1016/0042-6822(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Boulukos K., Leprince D., Dernis D., Lagrou C., Stehelin D. Identification in chicken macrophages of a set of proteins related to, but distinct from, the chicken cellular c-ets-encoded protein p54c-ets. EMBO J. 1986 Sep;5(9):2251–2256. doi: 10.1002/j.1460-2075.1986.tb04492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Bishop J. M. Structure and transcription of the cellular homolog (c-myb) of the avian myeloblastosis virus transforming gene (v-myb). J Virol. 1983 Apr;46(1):212–220. doi: 10.1128/jvi.46.1.212-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Haapala D. K., Robey W. G., Oroszlan S. D., Tsai W. P. Isolation from cats of an endogenous type C virus with a novel envelope glycoprotein. J Virol. 1985 Mar;53(3):827–833. doi: 10.1128/jvi.53.3.827-833.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurdic P., Benchaibi M., Gandrillon O., Samarut J. Transforming and mitogenic effects of avian leukemia virus E26 on chicken hematopoietic cells and fibroblasts, respectively, correlate with level of expression of the provirus. J Virol. 1987 Oct;61(10):3058–3065. doi: 10.1128/jvi.61.10.3058-3065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Integration of Moloney leukaemia virus into the germ line of mice: correlation between site of integration and virus activation. Nature. 1980 Oct 2;287(5781):456–458. doi: 10.1038/287456a0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Hartley J. W., Fredrickson T. N., Yetter R. A., Majumdar C., Cleveland J. L., Rapp U. R. Recombinant murine retroviruses containing avian v-myc induce a wide spectrum of neoplasms in newborn mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6868–6872. doi: 10.1073/pnas.83.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Nunn M., Weiher H., Bullock P., Duesberg P. Avian erythroblastosis virus E26: nucleotide sequence of the tripartite onc gene and of the LTR, and analysis of the cellular prototype of the viral ets sequence. Virology. 1984 Dec;139(2):330–339. doi: 10.1016/0042-6822(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Papas T. S., Bhat N. K., Chen T. T., Dubois G., Fisher R. J., Fujiwara S., Pribyl L. J., Sacchi N., Seth A., Showalter S. D. The ets genes in cells and viruses: implications for leukemias and other human diseases. Ann N Y Acad Sci. 1987;511:171–191. doi: 10.1111/j.1749-6632.1987.tb36246.x. [DOI] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Gesquière J. C., Stéhelin D., Ghysdael J. Mitogenic stimulation of thymocytes results in the calcium-dependent phosphorylation of c-ets-1 proteins. EMBO J. 1988 Apr;7(4):977–983. doi: 10.1002/j.1460-2075.1988.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribyl L. J., Watson D. K., McWilliams M. J., Ascione R., Papas T. S. The Drosophila ets-2 gene: molecular structure, chromosomal localization, and developmental expression. Dev Biol. 1988 May;127(1):45–53. doi: 10.1016/0012-1606(88)90187-x. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Papas T. S., Reddy E. S. erg, a human ets-related gene on chromosome 21: alternative splicing, polyadenylation, and translation. Science. 1987 Aug 7;237(4815):635–639. doi: 10.1126/science.3299708. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N., Papas T. S. The erg gene: a human gene related to the ets oncogene. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6131–6135. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi N., Watson D. K., Guerts van Kessel A. H., Hagemeijer A., Kersey J., Drabkin H. D., Patterson D., Papas T. S. Hu-ets-1 and Hu-ets-2 genes are transposed in acute leukemias with (4;11) and (8;21) translocations. Science. 1986 Jan 24;231(4736):379–382. doi: 10.1126/science.3941901. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Vennström B., Kahn P., Adkins B., Enrietto P., Hayman M. J., Graf T., Luciw P. Transformation of mammalian fibroblasts and macrophages in vitro by a murine retrovirus encoding an avian v-myc oncogene. EMBO J. 1984 Dec 20;3(13):3223–3229. doi: 10.1002/j.1460-2075.1984.tb02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Kozak C., Reeves R., Gearhart J., Nunn M. F., Nash W., Fowle J. R., 3rd, Duesberg P., Papas T. S. Conserved chromosomal positions of dual domains of the ets protooncogene in cats, mice, and humans. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1792–1796. doi: 10.1073/pnas.83.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Nunn M. F., Duesberg P. H., O'Brien S. J., Papas T. S. The ets sequence from the transforming gene of avian erythroblastosis virus, E26, has unique domains on human chromosomes 11 and 21: both loci are transcriptionally active. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7294–7298. doi: 10.1073/pnas.82.21.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Papas T. S. Molecular organization of the chicken ets locus. Virology. 1988 May;164(1):99–105. doi: 10.1016/0042-6822(88)90624-1. [DOI] [PubMed] [Google Scholar]