Abstract
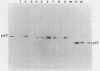
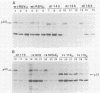
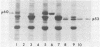
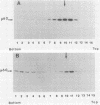
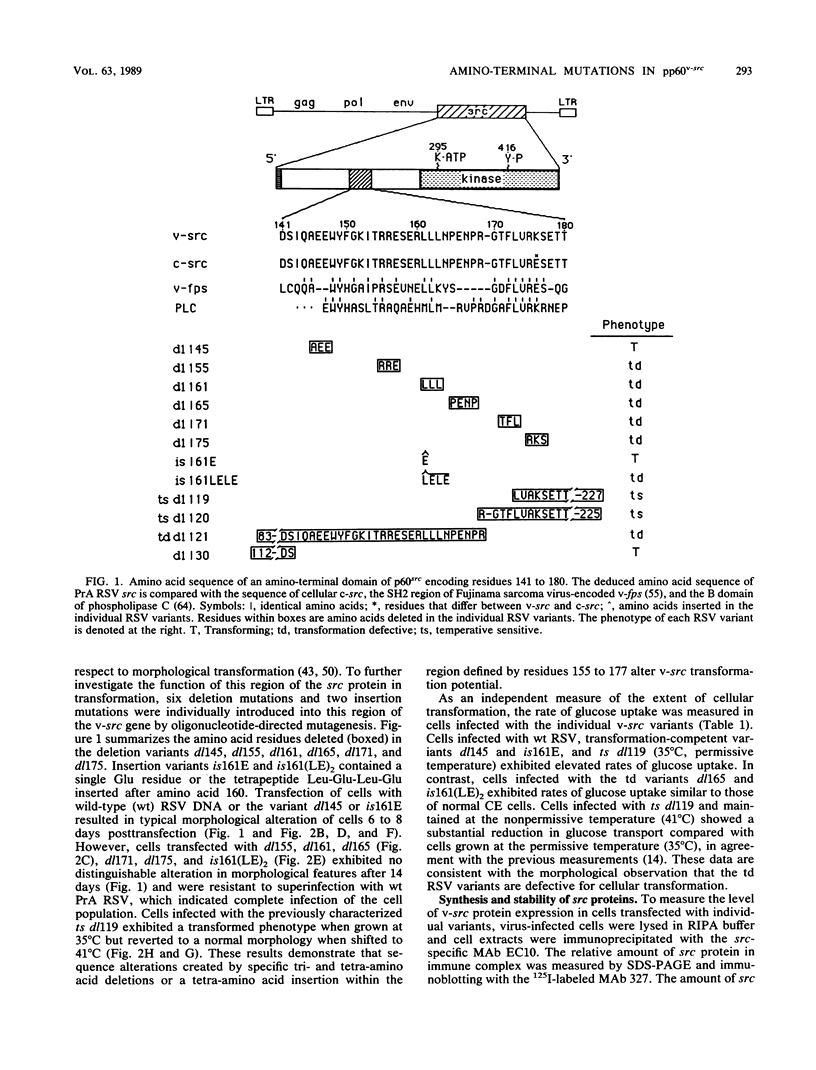
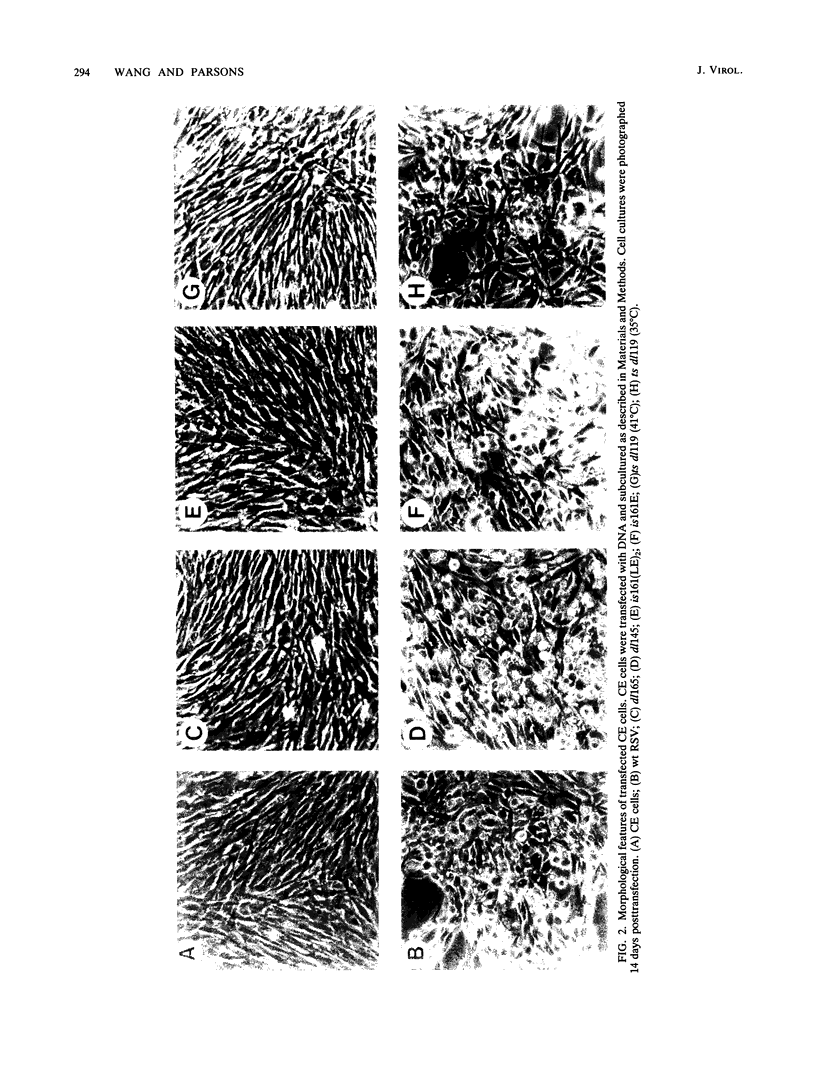
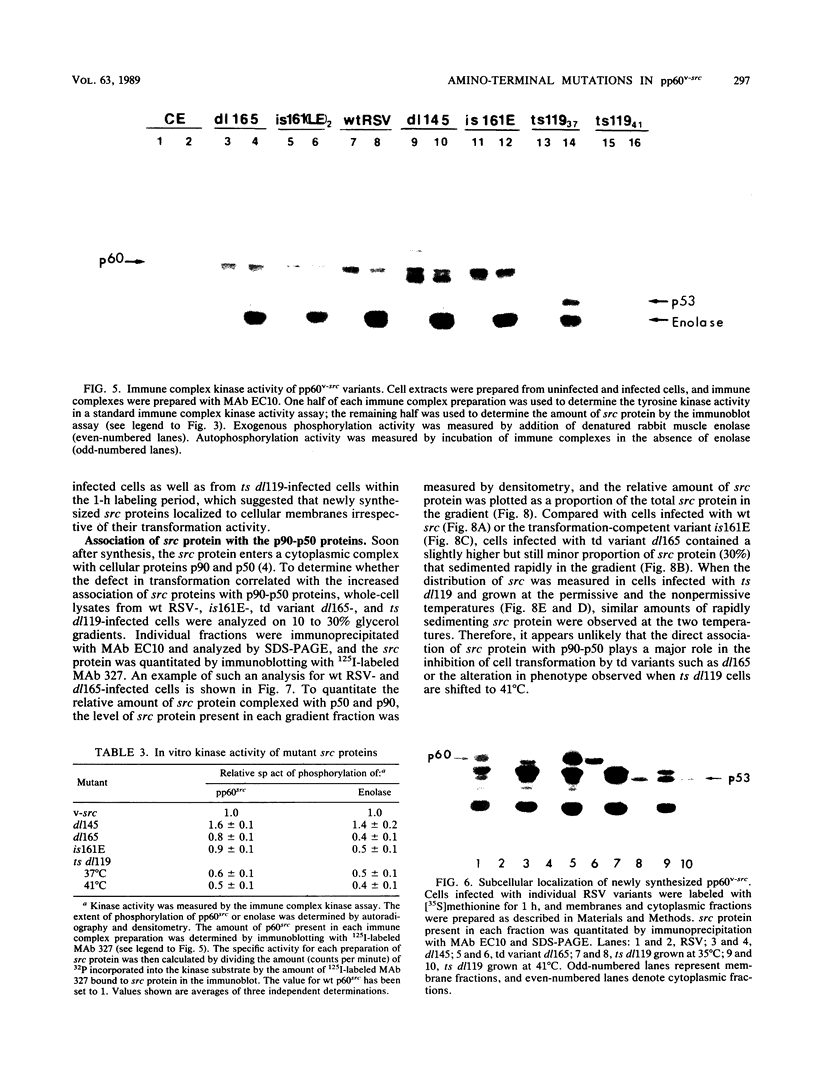
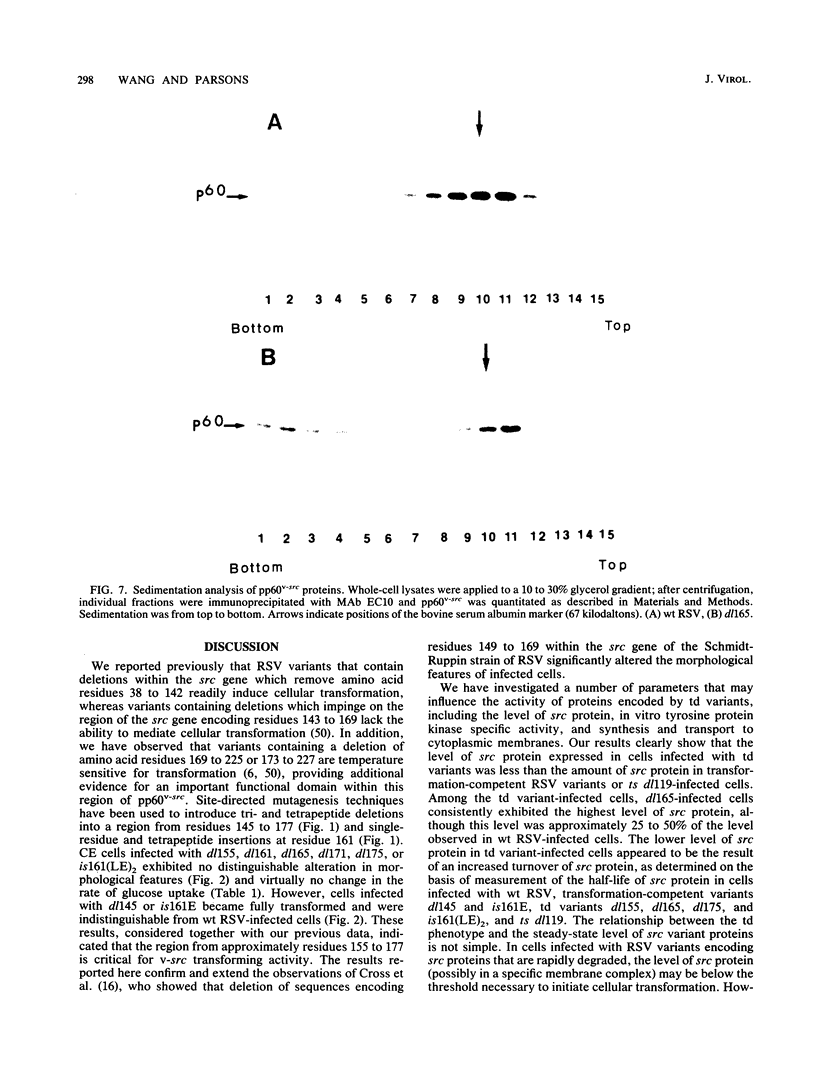
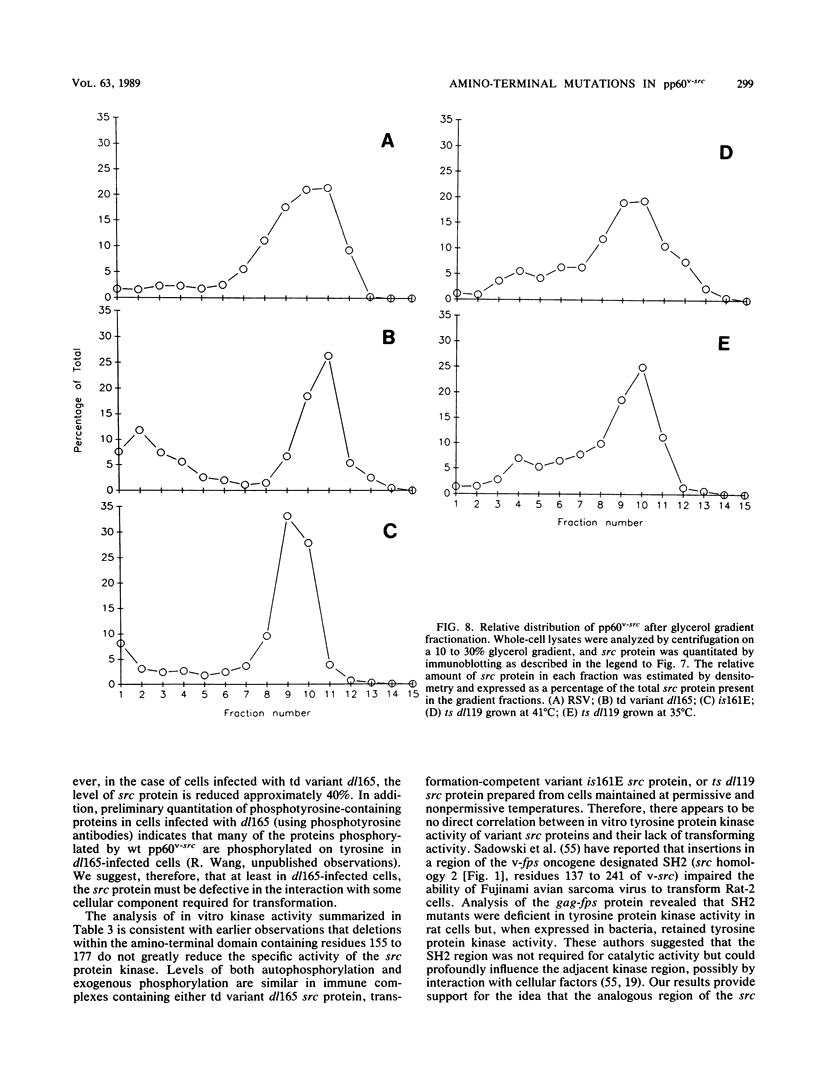
We previously showed (V. W. Raymond and J. T. Parsons, Virology 160:400-410, 1987) that variants of the Prague A strain of Rous sarcoma virus containing large deletions impinging on a region of the src gene encoding amino acid residues 143 to 169 were defective for transformation of chicken cells in culture. Here we report that introduction of small (tri-and tetrapeptide) deletions into a region of pp60v-src containing amino acid residues 155 to 175 was found to inactivate transformation. In addition, insertion of four, but not one, amino acid residues at position 161 also inhibited transformation. Biochemical analysis of the src proteins encoded by individual transformation-defective variants revealed that the structural alterations introduced into this domain had only marginal effects upon src tyrosine-specific protein kinase activity. However, the src proteins encoded by defective variants exhibited a significantly shorter half-life within the cell, although these proteins efficiently and rapidly associated with cellular membranes. Our results suggest that the structural domain encompassing residues 155 to 177 may influence the stability of pp60src in the cellular membrane, possibly via the interaction of src with a cellular membrane component(s) or substrate(s).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Brugge J. S., Darrow D. Analysis of the catalytic domain of phosphotransferase activity of two avian sarcoma virus-transforming proteins. J Biol Chem. 1984 Apr 10;259(7):4550–4557. [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Bryant D. L., Parsons J. T. Amino acid alterations within a highly conserved region of the Rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984 May;4(5):862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D., Parsons J. T. Site-directed mutagenesis of the src gene of Rous sarcoma virus: construction and characterization of a deletion mutant temperature sensitive for transformation. J Virol. 1982 Nov;44(2):683–691. doi: 10.1128/jvi.44.2.683-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Laugier D., Cross F. R., Jove R., Hanafusa T., Hanafusa H. The membrane-binding domain and myristylation of p60v-src are not essential for stimulation of cell proliferation. J Virol. 1987 May;61(5):1678–1681. doi: 10.1128/jvi.61.5.1678-1681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Cooper J., Nakamura K. D., Hunter T., Weber M. J. Phosphotyrosine-containing proteins and expression of transformation parameters in cells infected with partial transformation mutants of Rous sarcoma virus. J Virol. 1983 Apr;46(1):15–28. doi: 10.1128/jvi.46.1.15-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Phosphorylation of talin at tyrosine in Rous sarcoma virus-transformed cells. Mol Cell Biol. 1987 Jan;7(1):371–378. doi: 10.1128/mcb.7.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Sadowski I., Martin G. S., Pawson T. A conserved domain regulates interactions of the v-fps protein-tyrosine kinase with the host cell. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9064–9068. doi: 10.1073/pnas.84.24.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Fujita D. J., Bechberger J., Nedic I. Four Rous sarcoma virus mutants which affect transformed cell morphology exhibit altered src gene products. Virology. 1981 Oct 15;114(1):256–260. doi: 10.1016/0042-6822(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Fukami Y., Nakamura T., Nakayama A., Kanehisa T. Phosphorylation of tyrosine residues of calmodulin in Rous sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4190–4193. doi: 10.1073/pnas.83.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Development of anti-pp60src serum with antigen produced in Escherichia coli. J Virol. 1983 Jan;45(1):462–465. doi: 10.1128/jvi.45.1.462-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T. D., Radke K., Martin G. S. Tyrosine phosphorylation of a 50K cellular polypeptide associated with the Rous sarcoma virus transforming protein pp60src. Mol Cell Biol. 1982 Feb;2(2):199–206. doi: 10.1128/mcb.2.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Cooper J. A., Bretscher A., Hunter T. The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J Cell Biol. 1986 Feb;102(2):660–669. doi: 10.1083/jcb.102.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Rous sarcoma virus transforming protein lacking myristic acid phosphorylates known polypeptide substrates without inducing transformation. Cell. 1986 Apr 11;45(1):105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Neither arginine nor histidine can carry out the function of lysine-295 in the ATP-binding site of p60src. Mol Cell Biol. 1986 Mar;6(3):751–757. doi: 10.1128/mcb.6.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., Mardon G., Bishop J. M., Varmus H. E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988 Jun;8(6):2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Yoshida M. Small deletion in src of Rous sarcoma virus modifying transformation phenotypes: identification of 207-nucleotide deletion and its smaller product with protein kinase activity. J Virol. 1983 Jun;46(3):985–992. doi: 10.1128/jvi.46.3.985-992.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Weber M. J. Phosphorylation of a 36,000 Mr cellular protein in cells infected with partial transformation mutants of rous sarcoma virus. Mol Cell Biol. 1982 Feb;2(2):147–153. doi: 10.1128/mcb.2.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Wilkerson V., Parsons S. J. Structural and functional motifs of the Rous sarcoma virus src protein. Gene Amplif Anal. 1986;4:1–19. [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Sefton B. M. Phosphorylation of the transforming protein of Rous sarcoma virus: direct demonstration of phosphorylation of serine 17 and identification of an additional site of tyrosine phosphorylation in p60v-src of Prague Rous sarcoma virus. J Virol. 1986 Jul;59(1):73–81. doi: 10.1128/jvi.59.1.73-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F. Evidence the pp60src, the product of the Rous sarcoma virus src gene, undergoes autophosphorylation. J Virol. 1982 Jan;41(1):1–7. doi: 10.1128/jvi.41.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond V. W., Parsons J. T. Identification of an amino terminal domain required for the transforming activity of the Rous sarcoma virus src protein. Virology. 1987 Oct;160(2):400–410. doi: 10.1016/0042-6822(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L., Rosok M. J. Transformation parameters and pp60src localization in cells infected with partial transformation mutants of Rous sarcoma virus. Mol Cell Biol. 1983 Apr;3(4):731–746. doi: 10.1128/mcb.3.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Roth C. W., Richert N. D., Pastan I., Gottesman M. M. Cyclic AMP treatment of Rous sarcoma virus-transformed Chinese hamster ovary cells increases phosphorylation of pp60src and increases pp60src kinase activity. J Biol Chem. 1983 Sep 10;258(17):10768–10773. [PubMed] [Google Scholar]
- Sadowski I., Stone J. C., Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986 Dec;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Seki J., Owada M. K., Sakato N., Fujio H. Direct identification of phosphotyrosine-containing proteins in some retrovirus-transformed cells by use of anti-phosphotyrosine antibody. Cancer Res. 1986 Feb;46(2):907–916. [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Drees B., Kornberg T., Bishop J. M. The nucleotide sequence and the tissue-specific expression of Drosophila c-src. Cell. 1985 Oct;42(3):831–840. doi: 10.1016/0092-8674(85)90279-x. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., McGrath J. P., Levinson A. D. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol Cell Biol. 1985 Jul;5(7):1772–1779. doi: 10.1128/mcb.5.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Stoker A. W., Kellie S., Wyke J. A. Intracellular localization and processing of pp60v-src proteins expressed by two distinct temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1986 Jun;58(3):876–883. doi: 10.1128/jvi.58.3.876-883.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Verma I. M. Homology among oncogenes. Curr Top Microbiol Immunol. 1986;123:73–98. doi: 10.1007/978-3-642-70810-7_4. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Wilkerson V. W., Bryant D. L., Parsons J. T. Rous sarcoma virus variants that encode src proteins with an altered carboxy terminus are defective for cellular transformation. J Virol. 1985 Aug;55(2):314–321. doi: 10.1128/jvi.55.2.314-321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]