Abstract
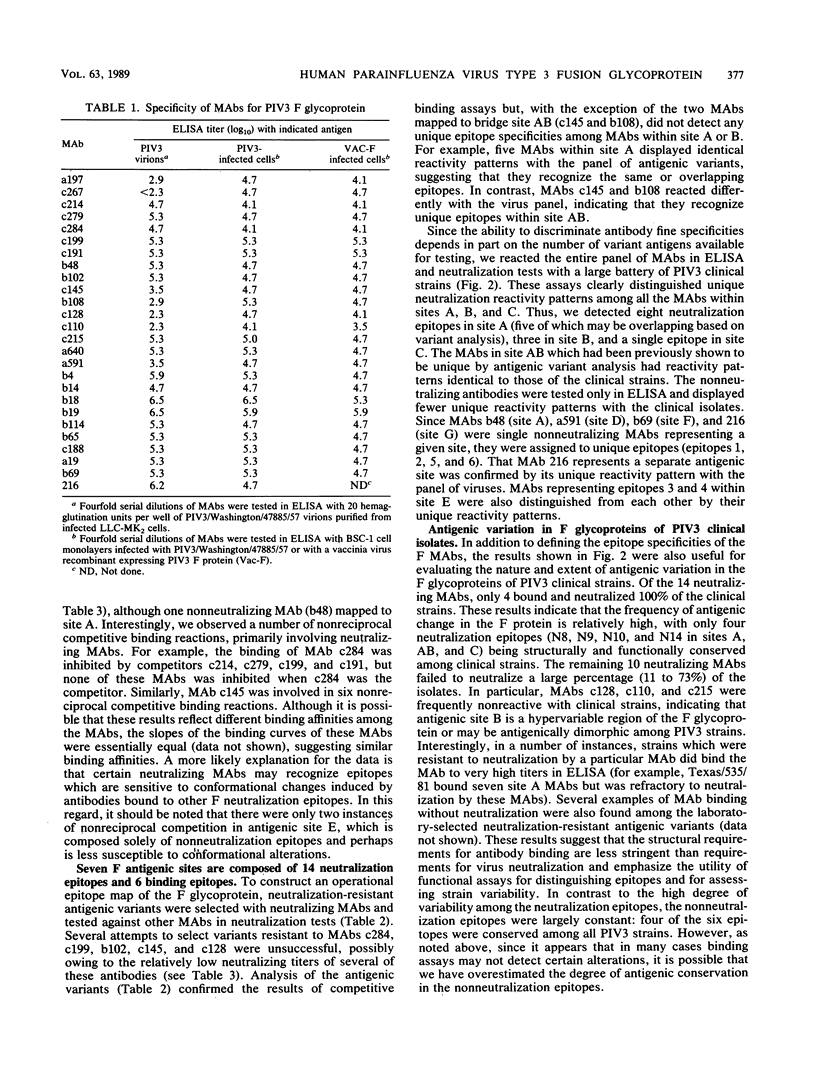
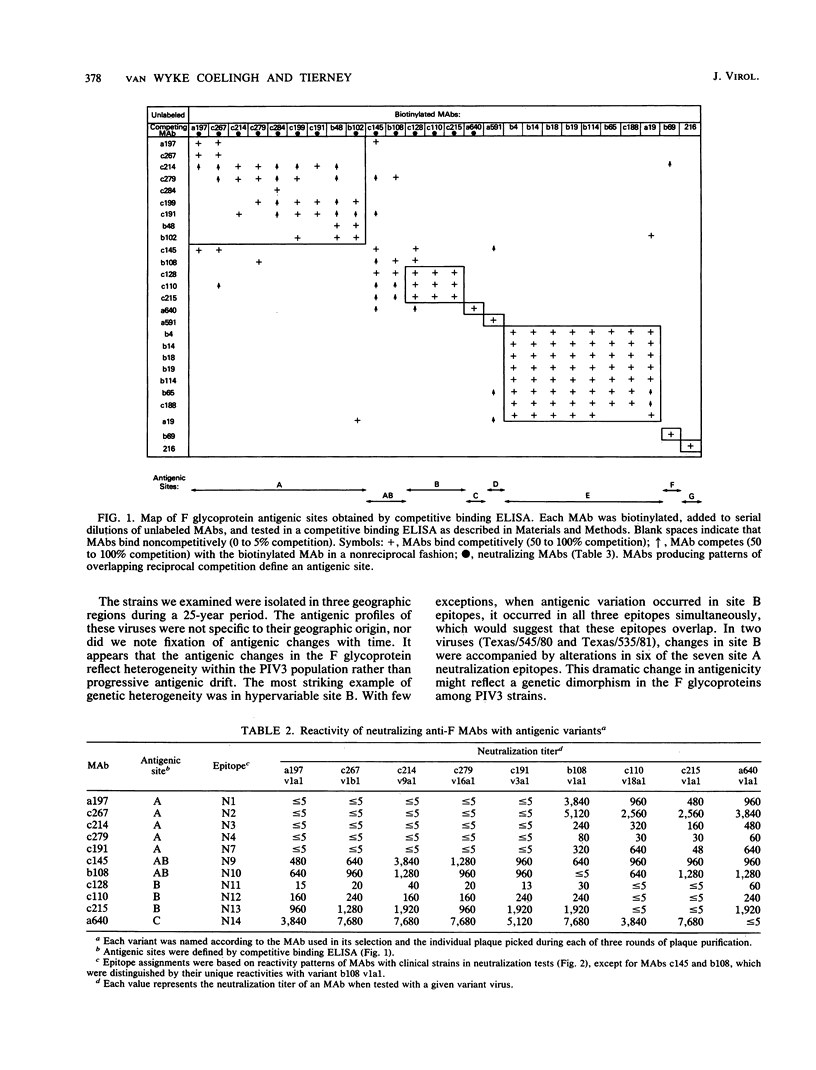
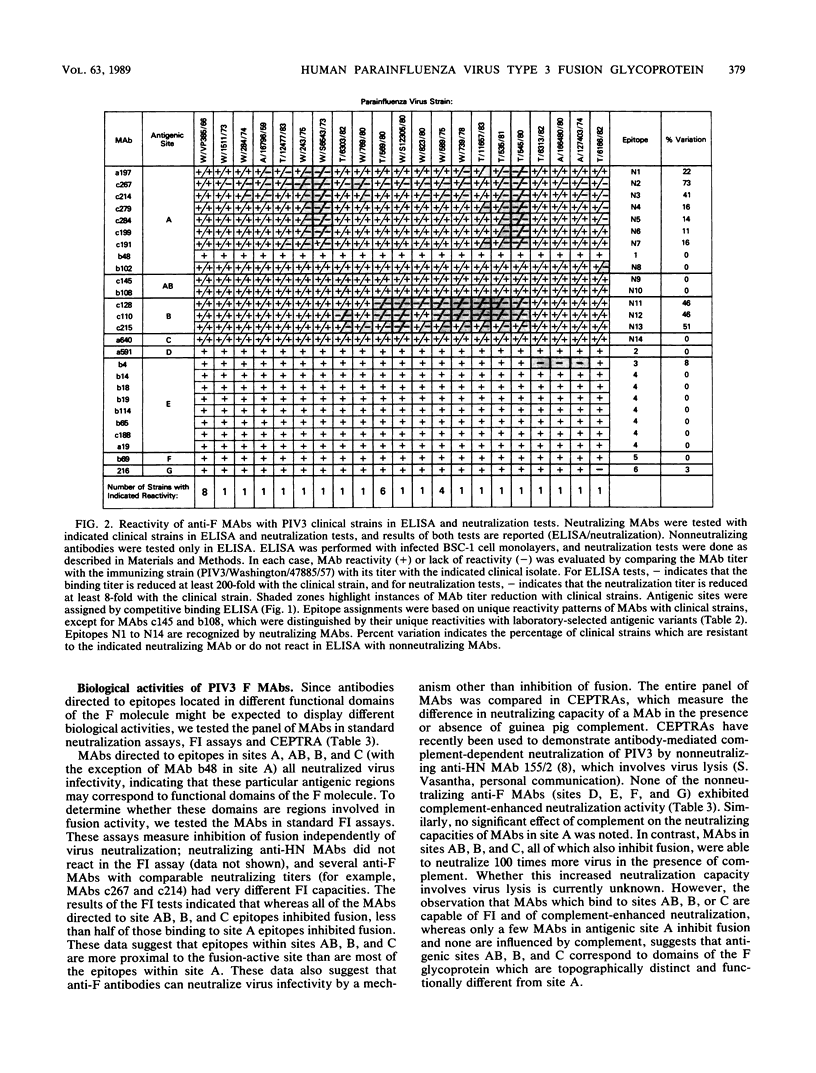
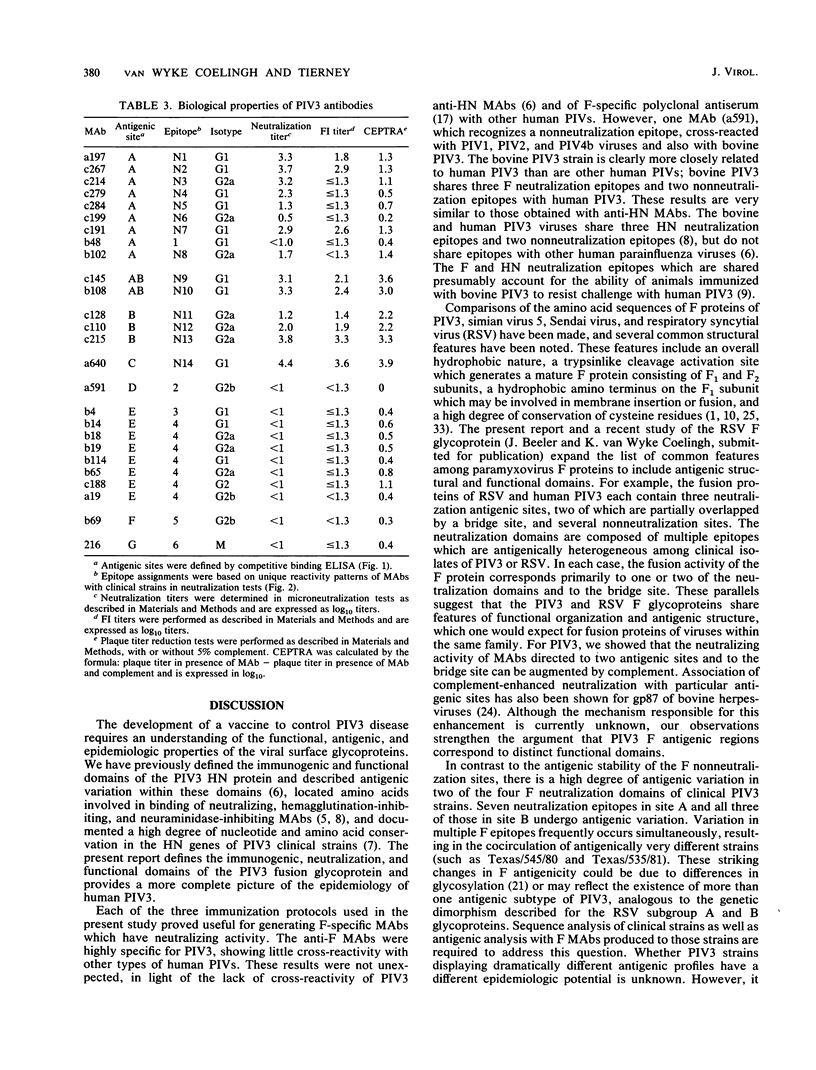
Twenty-six monoclonal antibodies (MAbs) (14 neutralizing and 12 nonneutralizing) were used to examine the antigenic structure, biological properties, and natural variation of the fusion (F) glycoprotein of human type 3 parainfluenza virus (PIV3). Analysis of laboratory-selected antigenic variants and of PIV3 clinical isolates indicated that the panel of MAbs recognizes at least 20 epitopes, 14 of which participate in neutralization. Competitive binding assays indicated that the 14 neutralization epitopes are organized into three nonoverlapping antigenic sites (A, B, and C) and one bridge site (AB) and that the 6 nonneutralization epitopes form four sites (D, E, F, and G). Most of the neutralizing MAbs were involved in nonreciprocal competitive binding reactions, suggesting that they induce conformational changes in other neutralization epitopes. Fusion-inhibition and complemented-enhanced neutralization assays indicated that antigenic sites AB, B, and C may correspond to functional domains of the F molecule. Our results indicated that antibody binding alone is not sufficient for virus neutralization and that many anti-F MAbs neutralize by mechanisms not involving fusion-inhibition. The degree of antigenic variation in the F epitopes of clinical strains was examined by binding and neutralization tests. It appears that PIV3 frequently develops mutations that produce F epitopes which efficiently bind antibodies, but are completely resistant to neutralization by these antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Buckley A., Gould E. A. Neutralization of yellow fever virus studied using monoclonal and polyclonal antibodies. J Gen Virol. 1985 Dec;66(Pt 12):2523–2531. doi: 10.1099/0022-1317-66-12-2523. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., COOK K., ANDREWS B. E., BELL J. A., REICHELDERFER T., KAPIKIAN A. Z., MASTROTA F. M., HUEBNER R. J. Newly recognized myxoviruses from children with respiratory disease. N Engl J Med. 1958 Jan 30;258(5):207–213. doi: 10.1056/NEJM195801302580502. [DOI] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Flamand A., Wiktor T. J., Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J Gen Virol. 1980 May;48(1):105–109. doi: 10.1099/0022-1317-48-1-105. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K. Sendai virus-erythrocyte membrane interaction: quantitative and kinetic analysis of viral binding, dissociation, and fusion. J Virol. 1986 Apr;58(1):87–95. doi: 10.1128/jvi.58.1.87-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Enhancement of membrane-fusing activity of sendai virus by exposure of the virus to basic pH is correlated with a conformational change in the fusion protein. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5862–5866. doi: 10.1073/pnas.79.19.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Scheid A., Choppin P. W. Activation of the Sendai virus fusion protein (f) involves a conformational change with exposure of a new hydrophobic region. J Biol Chem. 1981 Apr 10;256(7):3557–3563. [PubMed] [Google Scholar]
- Ito Y., Tsurudome M., Hishiyama M., Yamada A. Immunological interrelationships among human and non-human paramyxoviruses revealed by immunoprecipitation. J Gen Virol. 1987 May;68(Pt 5):1289–1297. doi: 10.1099/0022-1317-68-5-1289. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Frank A. L., Keitel W. A., Taber L. H., Glezen W. P. Acquisition of serum antibodies to specific viral glycoproteins of parainfluenza virus 3 in children. J Virol. 1984 Dec;52(3):828–832. doi: 10.1128/jvi.52.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G., Colman P. M. Crystals of antibodies complexed with influenza virus neuraminidase show isosteric binding of antibody to wild-type and variant antigens. Virology. 1987 Jan;156(1):181–184. doi: 10.1016/0042-6822(87)90451-x. [DOI] [PubMed] [Google Scholar]
- Long L., Portetelle D., Ghysdael J., Gonze M., Burny A., Meulemans G. Monoclonal antibodies to hemagglutinin-neuraminidase and fusion glycoproteins of Newcastle disease virus: relationship between glycosylation and reactivity. J Virol. 1986 Mar;57(3):1198–1202. doi: 10.1128/jvi.57.3.1198-1202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Norrby E., Utter G., Orvell C., Appel M. J. Protection against canine distemper virus in dogs after immunization with isolated fusion protein. J Virol. 1986 May;58(2):536–541. doi: 10.1128/jvi.58.2.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Honda E., Minetoma T., Kumagai T. Mechanisms of neutralization by monoclonal antibodies to different antigenic sites on the bovine herpesvirus type 1 glycoproteins. Virology. 1986 Apr 15;150(1):260–264. doi: 10.1016/0042-6822(86)90285-0. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Naeve C. W. The fusion glycoprotein of Sendai virus: sequence analysis of an epitope involved in fusion and virus neutralization. Virology. 1987 Apr;157(2):556–559. doi: 10.1016/0042-6822(87)90301-1. [DOI] [PubMed] [Google Scholar]
- Ray R., Compans R. W. Monoclonal antibodies reveal extensive antigenic differences between the hemagglutinin-neuraminidase glycoproteins of human and bovine parainfluenza 3 viruses. Virology. 1986 Jan 15;148(1):232–236. doi: 10.1016/0042-6822(86)90420-4. [DOI] [PubMed] [Google Scholar]
- Rydbeck R., Orvell C., Löve A., Norrby E. Characterization of four parainfluenza virus type 3 proteins by use of monoclonal antibodies. J Gen Virol. 1986 Aug;67(Pt 8):1531–1542. doi: 10.1099/0022-1317-67-8-1531. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Stanley J., Cooper S. J., Griffin D. E. Alphavirus neurovirulence: monoclonal antibodies discriminating wild-type from neuroadapted Sindbis virus. J Virol. 1985 Oct;56(1):110–119. doi: 10.1128/jvi.56.1.110-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Armstrong S. J., Dimmock N. J. Quantitative relationships between an influenza virus and neutralizing antibody. Virology. 1987 Aug;159(2):288–298. doi: 10.1016/0042-6822(87)90466-1. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Jorgensen E. D., Murphy B. R. Antigenic and structural properties of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3: sequence analysis of variants selected with monoclonal antibodies which inhibit infectivity, hemagglutination, and neuraminidase activities. J Virol. 1987 May;61(5):1473–1477. doi: 10.1128/jvi.61.5.1473-1477.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Murphy B. R. Nucleotide and deduced amino acid sequence of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses isolated from 1957 to 1983. Virology. 1988 Jan;162(1):137–143. doi: 10.1016/0042-6822(88)90402-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., London W. T., Murphy B. R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]



