Abstract
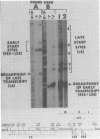
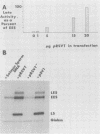
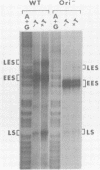
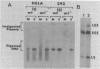
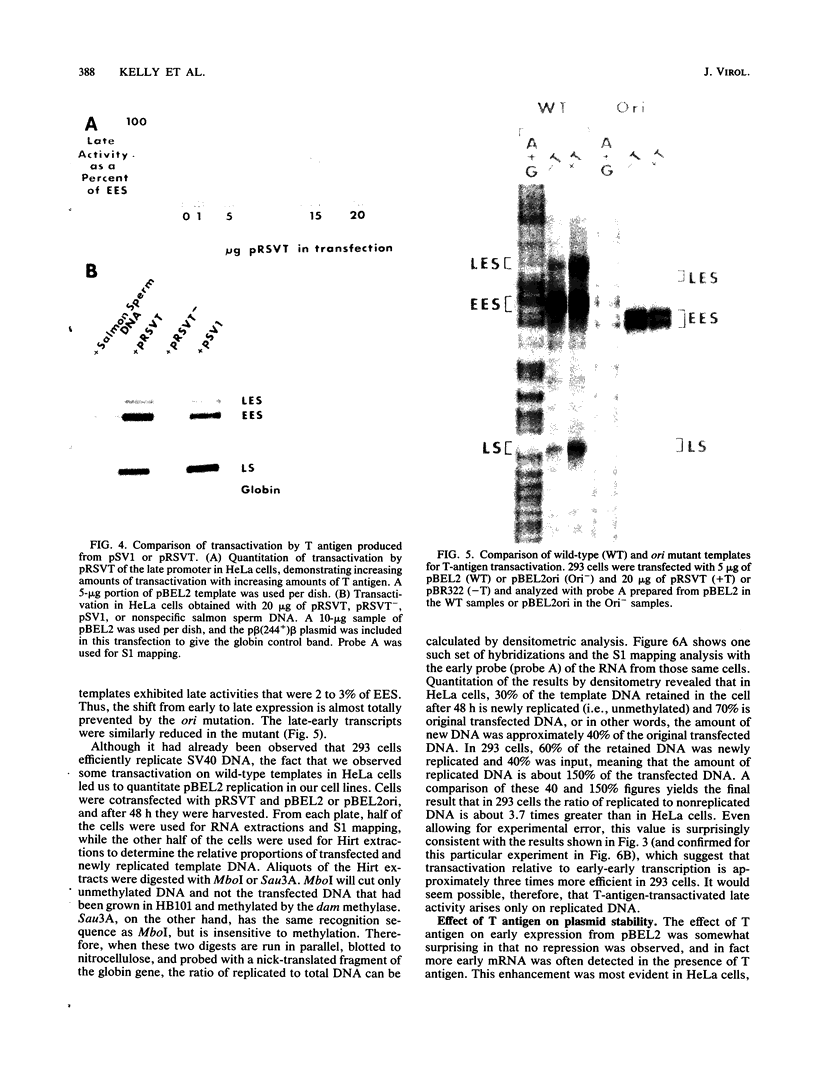
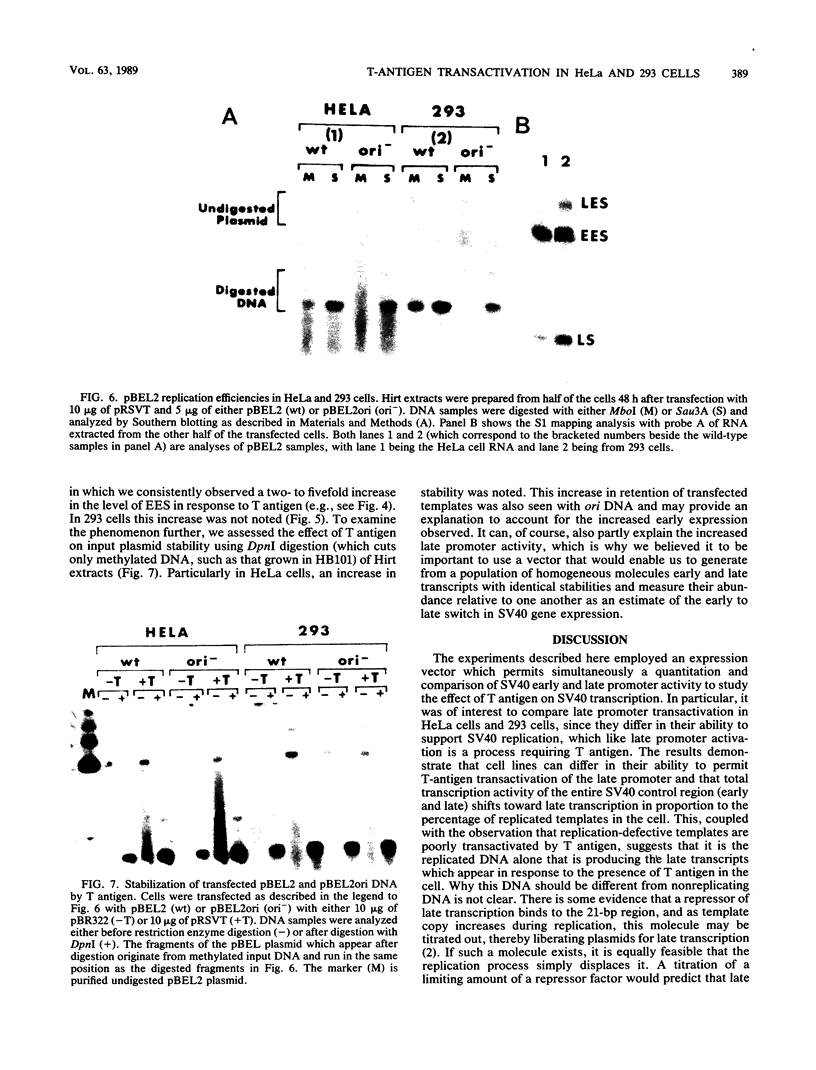
Transcription of the simian virus 40 (SV40) late promoter is strongly activated by SV40 T antigen. We were interested in examining this process in relation to other T-antigen functions such as replication and repression of early transcription. To quantitate the various T-antigen effects, we used a vector which has a promoterless beta-globin gene flanking the early and late sides of the SV40 promoter region. Following cotransfection with a plasmid encoding T antigen, transcription from the two promoters can be measured with a single S1 mapping probe and replication can be assayed by Southern blot analysis of DNA recovered in Hirt extracts. In this study, transactivation was examined in HeLa and 293 cells, since these cells differ in their ability to support SV40 replication. The strength of the late promoter relative to the early promoter was approximately three- to fourfold higher in 293 cells. Replication in 293 cells was also more efficient, by the same margin. In both cell lines, late promoter transactivation was barely detectable on replication-defective templates. Taken together, the results suggest that T-antigen activation of late transcription occurs only on replicated, or replicating, DNA. T antigen also activated the late-early start sites, and while in HeLa cells they were seen to be only 30% as strong as the late promoter, in 293 cells late and late-early activities were almost equal.
Full text
PDF
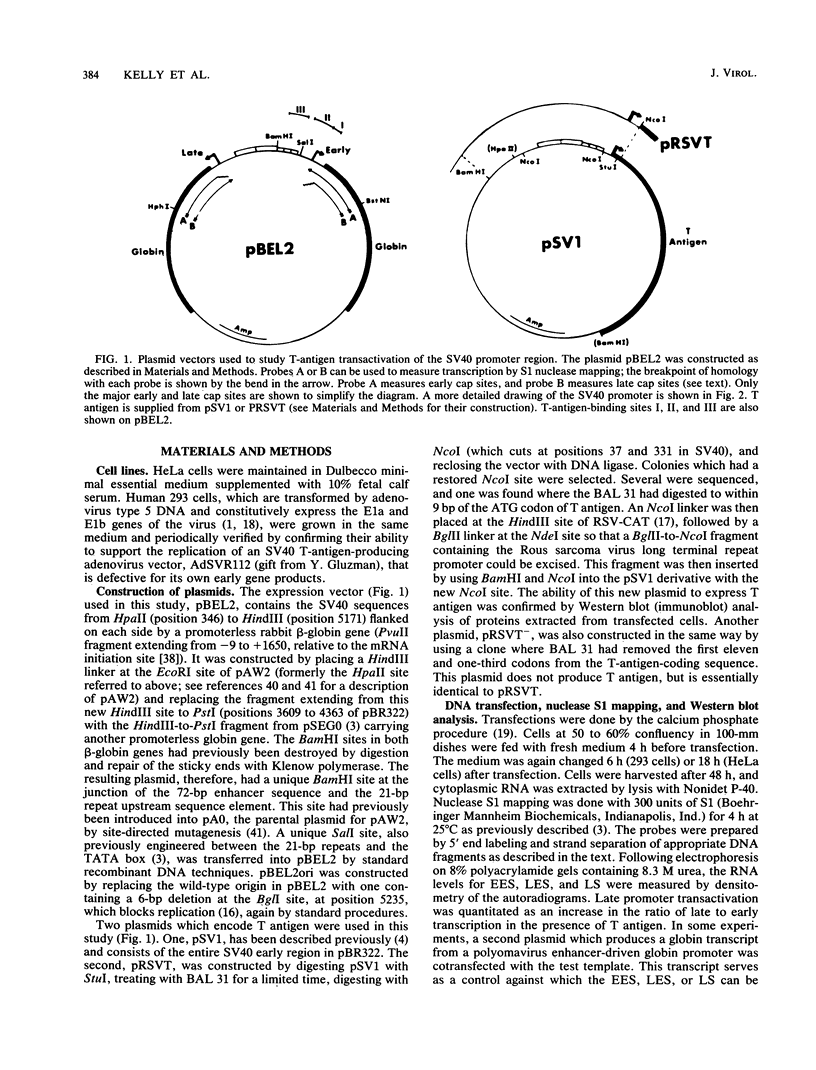

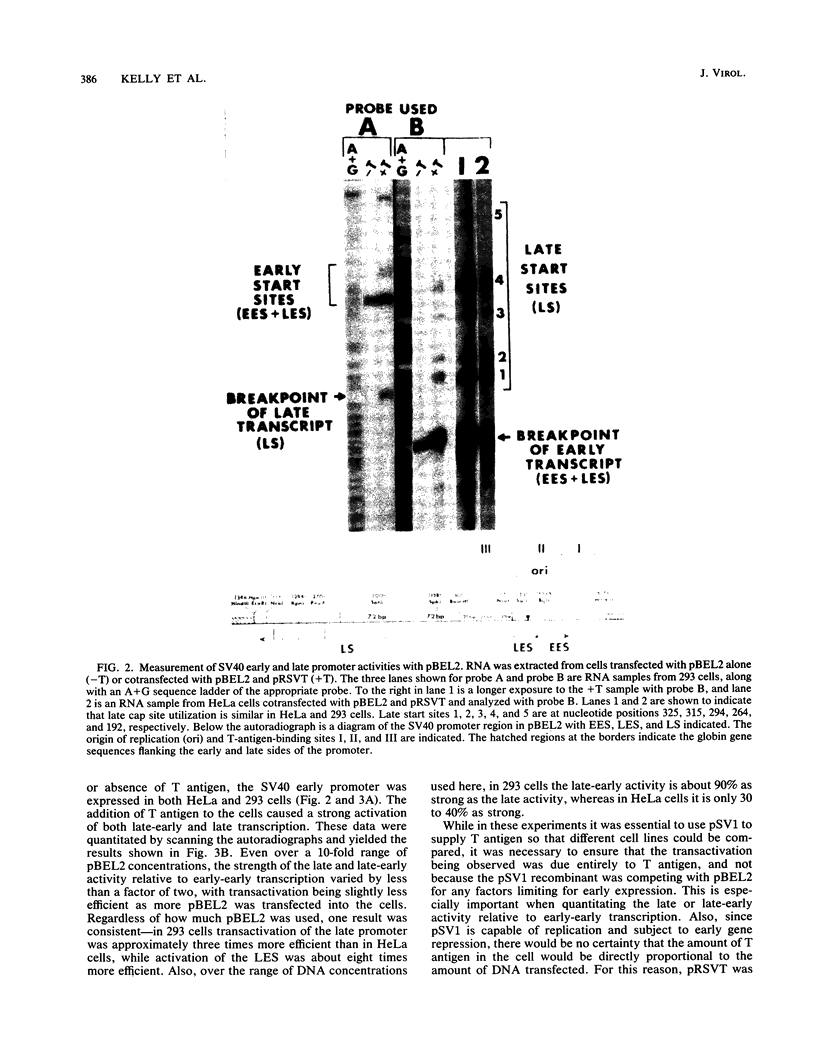
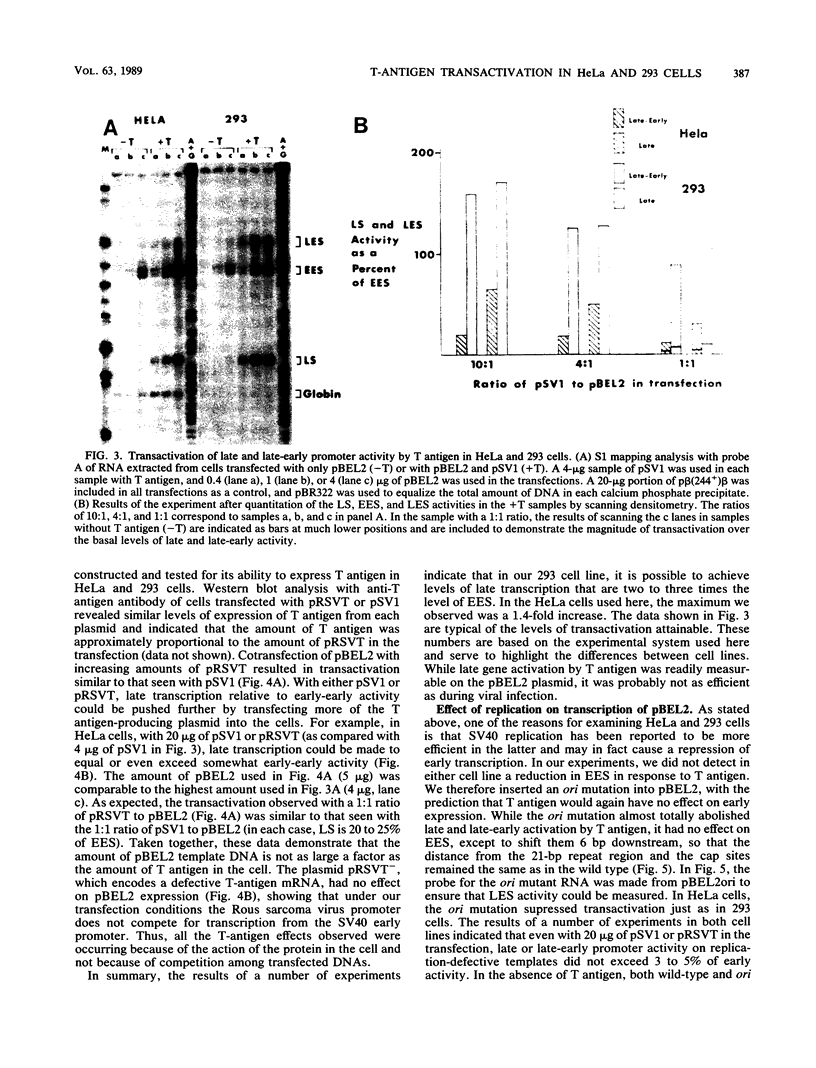


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello L., Guilfoyle R., Huebner K., Weinmann R. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology. 1979 Apr 30;94(2):460–469. doi: 10.1016/0042-6822(79)90476-8. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Picardi J. Activity of simian virus 40 late promoter elements in the absence of large T antigen: evidence for repression of late gene expression. J Virol. 1986 Nov;60(2):400–404. doi: 10.1128/jvi.60.2.400-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Brady J., Bolen J. B., Radonovich M., Salzman N., Khoury G. Stimulation of simian virus 40 late gene expression by simian virus 40 tumor antigen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2040–2044. doi: 10.1073/pnas.81.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Khoury G. trans Activation of the simian virus 40 late transcription unit by T-antigen. Mol Cell Biol. 1985 Jun;5(6):1391–1399. doi: 10.1128/mcb.5.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Loeken M. R., Khoury G. Interaction between two transcriptional control sequences required for tumor-antigen-mediated simian virus 40 late gene expression. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7299–7303. doi: 10.1073/pnas.82.21.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Berg P. Unusual regulation of simian virus 40 early-region transcription in genomes containing two origins of DNA replication. Mol Cell Biol. 1984 Sep;4(9):1915–1928. doi: 10.1128/mcb.4.9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Fromm M., Berg P. Complex regulation of simian virus 40 early-region transcription from different overlapping promoters. Mol Cell Biol. 1984 Sep;4(9):1900–1914. doi: 10.1128/mcb.4.9.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Bullock P., Murakami Y., Wobbe C. R., Weissbach L., Hurwitz J. Simian virus 40 (SV40) DNA replication: SV40 large T antigen unwinds DNA containing the SV40 origin of replication. Proc Natl Acad Sci U S A. 1987 Jan;84(1):16–20. doi: 10.1073/pnas.84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Regulatory mutants of simian virus 40. Effect of mutations at a T antigen binding site on DNA replication and expression of viral genes. J Mol Biol. 1982 Apr 15;156(3):531–548. doi: 10.1016/0022-2836(82)90265-0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P. Simian virus 40 early mRNA's contain multiple 5' termini upstream and downstream from a Hogness-Goldberg sequence; a shift in 5' termini during the lytic cycle is mediated by large T antigen. J Virol. 1981 Oct;40(1):224–240. doi: 10.1128/jvi.40.1.224-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grass D. S., Read D., Lewis E. D., Manley J. L. Cell- and promoter-specific activation of transcription by DNA replication. Genes Dev. 1987 Dec;1(10):1065–1074. doi: 10.1101/gad.1.10.1065. [DOI] [PubMed] [Google Scholar]
- Hansen U., Tenen D. G., Livingston D. M., Sharp P. A. T antigen repression of SV40 early transcription from two promoters. Cell. 1981 Dec;27(3 Pt 2):603–613. doi: 10.1016/0092-8674(81)90402-5. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Analysis of an activatable promoter: sequences in the simian virus 40 late promoter required for T-antigen-mediated trans activation. Mol Cell Biol. 1985 Aug;5(8):1859–1869. doi: 10.1128/mcb.5.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Calos M. P. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature. 1985 Sep 12;317(6033):169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- Lewis E. D., Manley J. L. Repression of simian virus 40 early transcription by viral DNA replication in human 293 cells. Nature. 1985 Sep 12;317(6033):172–175. doi: 10.1038/317172a0. [DOI] [PubMed] [Google Scholar]
- Lewton B. A., DeLucia A. L., Tegtmeyer P. Binding of simian virus 40 a protein to DNA with deletions at the origin of replication. J Virol. 1984 Jan;49(1):9–13. doi: 10.1128/jvi.49.1.9-13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher B. M., Dean D. H., Garro A. J. Fragmentation of Bacillus bacteriophage phi105 DNA by complementary single-stranded DNA in the cohesive ends of the molecule. J Virol. 1977 Aug;23(2):377–383. doi: 10.1128/jvi.23.2.377-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Matthes H., Wintzerith M., Chambon P. Transcription from the SV40 early-early and late-early overlapping promoters in the absence of DNA replication. EMBO J. 1983;2(9):1605–1611. doi: 10.1002/j.1460-2075.1983.tb01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]