Abstract
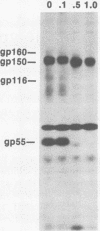
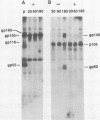
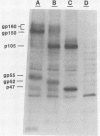
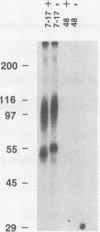
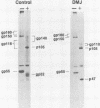
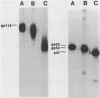
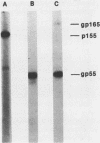
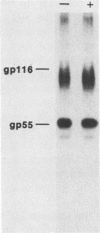
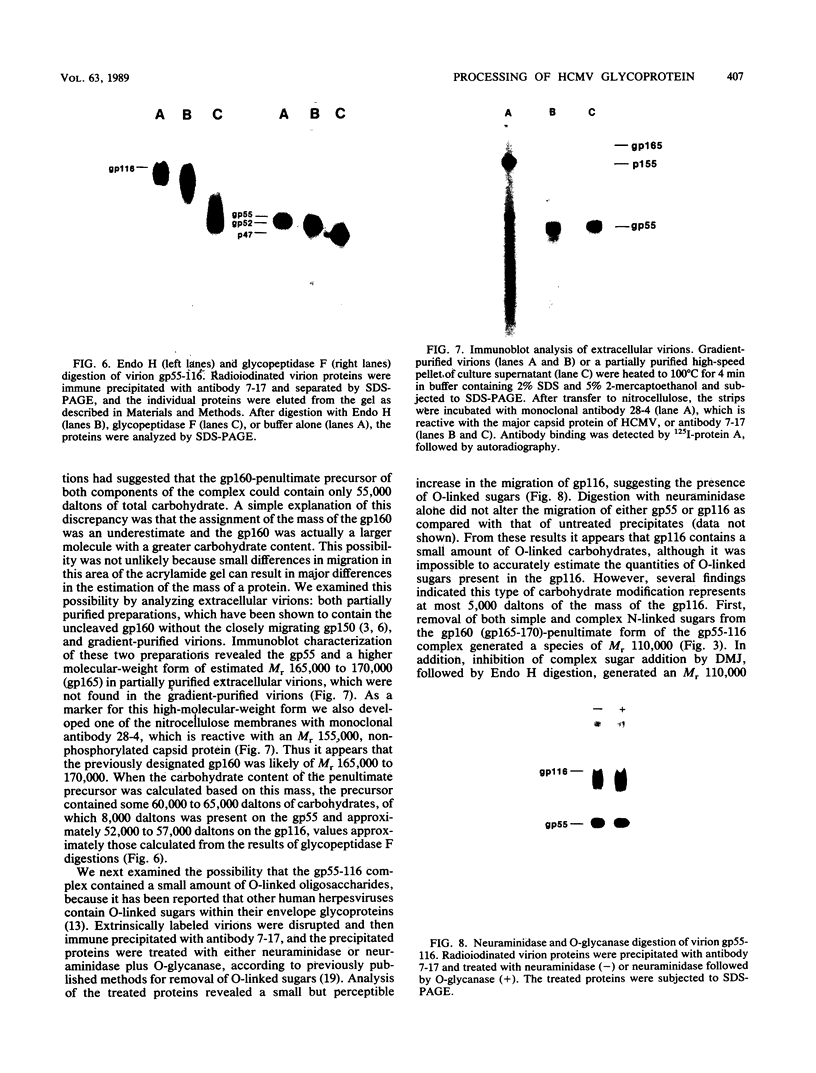
The processing pathway of the major envelope glycoprotein complex, gp55-116 (gB), of human cytomegalovirus was studied using inhibitors of glycosylation and endoglycosidases. The results of these studies indicated that the mature gp55-116 is synthesized by the addition of both simple and complex N-linked sugars to a nonglycosylated precursor of estimated Mr 105,000. In a rapid processing step, the Mr 105,000 precursor is glycosylated to a protein of Mr 150,000 (gp150) which contains only endoglycosidase H-sensitive sugar linkages. The gp150 is then processed relatively slowly to a Mr 165,000 to 170,000 species (gp165-170), which is then cleaved to yield the mature gp55-116. Monensin prevented the final processing steps of the gp150, including cleavage, suggesting that transport through the Golgi apparatus is required for complete processing. Digestion of the intracellular forms of this complex as well as the virion forms confirmed the above findings and indicated that the mature virion form of gp55 contains 8,000 daltons of N-linked sugars. The virion gp116 contains some 52,000 to 57,000 daltons of N-linked carbohydrates and approximately 5,000 daltons of O-linked sugars.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. A., Stagno S., Pass R. F. Natural history of perinatal cytomegaloviral infection. Ciba Found Symp. 1979;(77):125–147. doi: 10.1002/9780470720608.ch9. [DOI] [PubMed] [Google Scholar]
- Benko D. M., Gibson W. Primate cytomegalovirus glycoproteins: lectin-binding properties and sensitivities to glycosidases. J Virol. 1986 Sep;59(3):703–713. doi: 10.1128/jvi.59.3.703-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Human cytomegalovirus virion-associated protein with kinase activity. J Virol. 1986 Jul;59(1):185–188. doi: 10.1128/jvi.59.1.185-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Identification of a 65 000 dalton virion envelope protein of human cytomegalovirus. Virus Res. 1985 Dec;4(1):31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986 Apr;58(1):185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Vugler L., Stephens E. B. Induction of complement-dependent and -independent neutralizing antibodies by recombinant-derived human cytomegalovirus gp55-116 (gB). J Virol. 1988 Sep;62(9):3309–3318. doi: 10.1128/jvi.62.9.3309-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar G. H., Oram J. D. Characterization of the human cytomegalovirus envelope glycoproteins. J Gen Virol. 1984 Nov;65(Pt 11):1991–2001. doi: 10.1099/0022-1317-65-11-1991. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Gehrz R. C., Stinski M. F. Characterization of a human cytomegalovirus glycoprotein complex (gcI). J Gen Virol. 1988 Jun;69(Pt 6):1205–1215. doi: 10.1099/0022-1317-69-6-1205. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Rasmussen L., Gehrz R. C., Stinski M. F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol. 1988 Mar;62(3):875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Namazue J., Campo-Vera H., Kitamura K., Okuno T., Yamanishi K. Processing of virus-specific glycoproteins of varicella zoster virus. Virology. 1985 May;143(1):252–259. doi: 10.1016/0042-6822(85)90112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B., Sullivan C., Sarnow P., Thomas R., Bricout F., Nicolas J. C., Fleckenstein B., Levine A. J. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology. 1984 Jan 30;132(2):325–338. doi: 10.1016/0042-6822(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Tatsuno M., Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984 Nov;139(1):73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. O-linked glycosylation of retroviral envelope gene products. J Virol. 1988 Mar;62(3):1016–1021. doi: 10.1128/jvi.62.3.1016-1021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson R., Merigan T. C. Viral polypeptides detected by a complement-dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985 Aug;55(2):274–280. doi: 10.1128/jvi.55.2.274-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Nelson M., Neff M., Merigan T. C., Jr Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988 Apr;163(2):308–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Adams M. R., Rabin H. Glycosylation pathways of two major Epstein-Barr virus membrane antigens. Virology. 1983 May;127(1):168–176. doi: 10.1016/0042-6822(83)90381-1. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Effect of tunicamycin and monensin on biosynthesis, transport, and maturation of bovine herpesvirus type-1 glycoproteins. Virology. 1985 May;143(1):104–118. doi: 10.1016/0042-6822(85)90100-X. [DOI] [PMC free article] [PubMed] [Google Scholar]