Abstract
A monoclonal antibody specific for the empty conformation of class II MHC molecules revealed the presence of abundant empty molecules on the surface of spleen- and bone marrow-derived dendritic cells (DC) among various types of antigen-presenting cells. The empty class II MHC molecules are developmentally regulated and expressed predominantly on immature DC. They can capture peptide antigens directly from the extracellular medium and present bound peptides to antigen-specific T lymphocytes. The ability of the empty cell-surface class II MHC proteins to bind peptides and present them to T cells without intracellular processing can serve to extend the spectrum of antigens able to be presented by DC, consistent with their role as sentinels in the immune system.
MHC class II molecules bind peptides derived from proteins that have entered the endocytic pathway and present them at the cell surface for interaction with CD4+ T cells (1). MHC class II molecules are constitutively expressed by B cells, macrophages, and dendritic cells (DC), the “professional” antigen-presenting cells of the immune system, and their expression can be induced on many other cell types by treatment with IFN-γ. Pathways for intracellular assembly and folding of class II MHC proteins, for proteolytic processing of antigens, and for peptide loading onto class II MHC proteins, have been characterized in detail (2). In B cells, nascent class II MHC αβ heterodimers rapidly associate in the endoplasmic reticulum with the invariant chain (Ii) chaperonin protein (3, 4). MHC-Ii complexes transport to early endosomal compartments under the influence of a dileucine sorting signal on the Ii cytoplasmic tail (3), either directly from the trans-Golgi network or alternately after transient expression on the cell membrane and rapid internalization (5). MHC-Ii complexes traverse the endocytic pathway from early to late endosomes and finally into lysosomes (6). In these compartments, bound Ii is degraded by cathepsins and other endosomal proteases, leaving a small fragment (CLIP) bound in the MHC peptide-binding groove (7, 8). The CLIP fragment is exchanged for peptides generated in the endosome as a result of proteolytic degradation, in a process catalyzed by the endosomal-resident peptide exchange factor DM (9). Finally, MHC-peptide complexes are transported to the B-cell surface. MHC molecules that have not received peptide are functionally inactivated, retained within the cell, and degraded (10). Another pathway for antigen loading has been observed that is independent of Ii and CLIP. Class II MHC molecules carry a leucine motif in the cytoplasmic region of the β chain (11), and MHC-peptide complexes can be recycled from the cell surface back into the endosomes for antigen loading by peptide exchange (12).
These pathways for MHC trafficking and antigen loading have been characterized mostly in B cells and used as general models for class II-mediated antigen processing and presentation in other cell types. However, several recent studies have reported profound differences in the regulation and trafficking of class II MHC molecules in DC relative to B cells. Class II MHC expression in all cell types is regulated by the class II transcription transactivator (CIITA) and the transcription factor complex RFX. CIITA is differentially expressed in DC and B cells, leading to differential regulation of MHC synthesis (13). Moreover, in transgenic mice deficient in CIITA (14, 15) or RFX5 (16), residual class II expression can still be observed in DC but not in B cells. Differences in MHC intracellular transport also have been reported for DC relative to B cells. In DC from mice deficient in Ii, normal levels of cell-surface class II MHC are expressed, whereas in B cells, expression is significantly reduced (17). In DC, the majority of nascent class II MHC-Ii complexes transport to the cell surface en route to endocytic compartments, whereas in B cells this is a minor pathway (6). Finally, class II MHC can access a nonacidic early endosomal compartment used for antigen storage in DC that has not been observed in B cells (18).
These findings begin to provide some explanations for the different antigen presentation functions of B cells and DC and in particular for the key role played by DC in priming the immune response and in presenting antigen to naïve T cells. In this report, we present evidence for an extracellular antigen-loading and -presentation pathway that appears to be active in DC, in addition to pathways characterized in B cells. We find that immature DC express abundant empty class II MHC molecules on their cell surface, and that such molecules are able to collect peptide antigen from the extracellular milieu for presentation to T cells. These molecules may participate in the unique antigen presentation functions of DC.
Methods
Recombinant MHC Molecules.
MHC proteins were produced by baculovirus-mediated expression in Sf9 insect cells. For HLA-DR1, soluble extracellular domains and full-length membrane glycoproteins were produced as described (19). For IAs, DNA fragments encoding signal sequence and extracellular domains IAsα (EDDIEA … SELTET) and IAsβ (GDSETL … ESARSK) subunits were amplified by PCR and cloned into pFastBac1 (GIBCO/BRL) to produce recombinant bacmids used to transfect Sf9 cells. Recombinant baculovirus clones were isolated and used to infect Sf9 cells in Sf900 medium (GIBCO/BRL), as described for HLA-DR1 (19). Flow cytometry experiments were performed 5 days after infection. In some experiments, 10 μM peptide was added to the medium 3 days after infection, as noted. An alternate expression system was used for production of empty or peptide-loaded DR1 in Escherichia coli (20). Briefly, HLA-DR1 extracellular domains were expressed individually as insoluble inclusion bodies, isolated by denaturing ion exchange chromatography, and refolded in vitro with or without peptide, as described (20).
Flow Cytometry.
Primary antibodies used in cytometry, mouse monoclonals Y3P (anti-IAs,u,f) (10, 21), 10–2.16 (anti-IAs,k,u,f) (10, 21), 10–3.6.2 (anti-IAs,k) (22), KL-304 (anti-IAs,k,u,f β 57–68) (23), 11–5.2 (anti-IAk) (24), MK-S4 (anti-IAs) (25), hamster monoclonal CD 11c (26), and rat monoclonal DEC-205 (27) were produced in hybridomas (American Type Culture Collection) and purified by ammonium sulfate precipitation and protein A or protein G chromatography (28). Purified mouse monoclonal antibodies MRC-OX3 and MRC-OX6 (anti-IA) (29) were purchased from Serotech, and species- and isotype-matched control monoclonal antibodies from PharMingen. For flow cytometry, cells were incubated on ice with saturating amounts of primary antibody for 30 min in PBS (150 mM NaCl/10 mM Na-phosphate/12 mM NaN3, pH 7.2) containing 1 mg/ml BSA, and then washed, incubated with fluorescein- or phycoerythrin-conjugated (Fab′)2 secondary antibody (Jackson ImmunoResearch) that had been preabsorbed with normal serum, washed again, and analyzed immediately by using a FACscalibur flow cytometer (Becton Dickinson). Fluorescence values were converted to numbers of receptors per cell by using calibrated flow cytometry beads (QIFIKit, Dako). In some experiments, Fcγ receptor binding was blocked by preincubation with 1 μg of rat monoclonal antibody CD16/CD32 (PharMingen).
Cell Culture.
Mouse strains SJL/J (IAs), B10.S (IAs), B10.BR (IAk), C3H (IAk), PL/J (IAu), and B10.M (IAf) were obtained from Jackson (Bar Harbor, ME). MHC class II−/− mice (30) were obtained from J. Strominger, Harvard University. Splenic DC were derived by selective attachment and negative antibody selection as previously described (31), and were maintained in DMEM supplemented with 5% fetal bovine serum/2 mM glutamine/nonessential amino acids/1 mM sodium pyruvate/20 mM Hepes buffer (cDMEM). Cultures were supplemented with 10 ng/ml mouse recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems) every 4 d. Under these conditions, most cells had the phenotype of intermediate DC, although mature DC were observed. The fraction of mature DC increased with time in culture. Similar staining results were obtained with fresh noncultured DC isolated from spleen by BSA gradients (28). Bone marrow DC were established by ex vivo differentiation of precursor cells as described (28). Cultures were maintained in cDMEM supplemented every 2 days with 10 ng/ml GM-CSF. B cells, T cells, and granulocytes were removed by using rat monoclonal antibodies (PharMingen) B220/CD45R, CD90.1/Thy 1.1, CD90.2/Thy 1.2, and Ly6GGR-1/RB6–8C5, in conjunction with magnetic beads coated with sheep anti-rat IgG (Dynal, Great Neck, NY), first on initial isolation from bone marrow and again immediately before staining. To obtain mature bone marrow-derived DC, cells were collected, washed, and subcultured for 1–2 days in the absence of GM-CSF. In some experiments, subcultured DC were further differentiated by treatment with 100 units/ml mouse recombinant tumor necrosis factor α (TNFα) (PharMingen) for 1–2 d. Splenic and peritoneal B cells and macrophages were obtained by adherence and negative selection protocols (28). In some experiments (Fig. 3 and Fig. 5 D and E), B cells and macrophages were treated with 15 ng/ml (bacterial) lipopolysaccharide for 48 hr to increase MHC class II expression to levels comparable to those of DC.
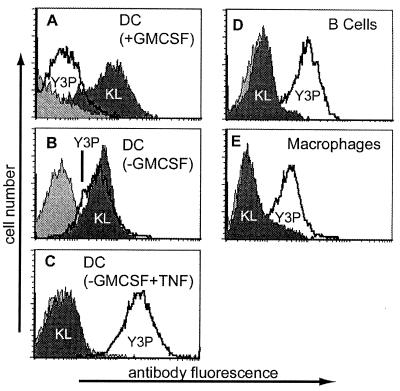
Figure 3.
Immature DC express empty cell-surface class II MHC molecules. Flow cytometry of various professional antigen-presenting cells by using antibodies KL-304 (shaded), Y3P (unshaded), and control IgG (light shading), for bone marrow-derived DC in various developmental states (A–C), B-cell blasts (D), or macrophages (E). The relative amount of KL-304 to Y3P staining varies with developmental state for bone marrow-derived DC. KL-304 (Y3P) staining corresponds to the following numbers of molecules per cell: DC + GM-CSF, 259,000 (38,000); DC-GM-CSF, 243,000 (304,000); DC-GM-CSF + TNF, <3,600 (>470,000); B cells <6,000 (>470,000), macrophages 19,000 (428,000).
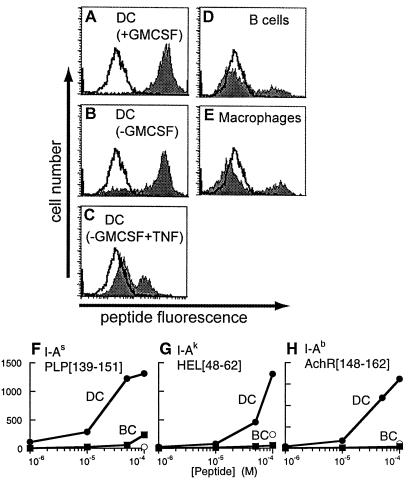
Figure 5.
Peptide binding by empty cell-surface class II MHC molecules. (A–E) Peptide binding to cell-surface IAs on bone marrow-derived DC in various developmental states (A–C), B-cell blasts (D), or macrophages (E), by using 100 μM fluorescent antigenic peptide PLP[139–151] (shaded) or control peptide PLP[39–50] (unshaded), detected by flow cytometry. Proportion of cells staining positive for surface peptide binding: DC (+GM-CSF) 99%, mature DC (−GM-CSF) 81%, DC (−GM-CSF, +TNF) 29%, macrophages 23%, B-cell blasts 21%. (F–H) Concentration dependence of antigenic peptide binding to splenic DC (filled circles) and B-cell blasts (filled squares) and of control peptide binding to splenic DC (open circles). (F) PLP[139–151] binding to DC and B cells from SJL (IAs) mice with nonbinding control peptide PLP[39–50], (G) HEL[48–62] binding to B10.Br (IAk) cells with control peptide OVA[323–336], and (H) AchR[148–162] binding to C57/Bl6 (IAb) cells with control peptide MBP[1–11].
KL304-Binding and Specificity Assays.
Peptide analogs of the KL-304 epitope (AEYYNKQYLEQT) (23) were synthesized with fluorenylmethoxycarbonyl (Fmoc) protection and purified by reverse-phase chromatography by using standard methods. KL-304 epitope mapping was performed by competitive ELISA by using immobilized IAsβ and various concentrations of epitope analog peptides. A sandwich ELISA assay (20) was used for determination of the relative binding of empty and peptide-loaded HLA-DR1 from E. coli, with immobilized KL-304 for antigen capture, and rabbit anti-DR1 serum with peroxidase-labeled goat anti-rabbit serum for antigen detection. For plasmon resonance, the Fab fragment of KL-304 was prepared by papain digestion (28) and was coupled to a CM5 chip (Pharmacia Biosensor) through amine groups by using N-hydroxysuccinimide and N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide. Attempts to couple the intact antibody through amine groups resulted in loss of activity. Samples were applied to KL-304 and control (no antibody) flowcells at 5 μl/min in PBS with 0.005% P20 surfactant, and for each sample the control trace was subtracted from the KL-304 trace.
Cell-Surface Peptide-Binding Assay.
IA binding peptides PLP [139–151] (HSLGKWLGHPDKF) (32), AchR [148–162] (IWTYDGTKVSISPES) (33), MBP [1–11] (AcASQYRPSQRHG] (34), HEL [48–62] (DGSTDYGILQINSRW) (35), control peptides PLP[35–50] (GHEALTGTEKLIETYF) (32), OVA [323–336] (ISQAVHAAHAEINE), and HLA-DR1 binding peptides HA[306–318] (PKYVKQNTLKLAT) (36), YAK (AAYAAAAAAKAAA) (37), Min 4 (Ac-YRAL-NH2) (37), and CLIP (KMRMATPLLMQALPM) (38), were synthesized with Fmoc protection and purified by reverse-phase chromatography by using standard methods. For fluorescent labeling, peptides were prepared with a 13-residue N-terminal linker, ɛGGGGSCRRGGGGS-, where ɛ is amino-caproic acid, and labeled at the N terminus by using 10-fold molar excess FITC in 1:1 dimethylformamide/N-methylmorpholine before side-chain deprotection. For binding assays, cells were washed, incubated with various peptide concentrations in PBS containing 1% BSA for 2 hr at 37°C, and washed three times before determination of bound peptide by flow cytometry.
T-Cell Proliferation.
A T-cell line (SP11) restricted by IAs and specific for PLP [139–151] was raised and maintained as described (39). For assay of antigen-specific T-cell activation, 5 × 104 irradiated splenic DC or 5 × 105 irradiated splenic B cells were incubated with different concentrations of antigenic peptide PLP [139–151] (0.08–50 μg/ml) and 1 × 105 SP11 T cells, at 37°C and 5% CO2, with [3H]thymidine (1 μCi) added after 48 hr of culture and incorporated radioactivity determined as described (28). For antibody inhibition, 1 μg/well of MRC-OX6, KL-304, or isotype-matched (IgGγ1or IgGγ2b) control antibodies were added during the initial incubation with antigen.
Results
KL-304 Specifically Binds Empty MHC Molecules.
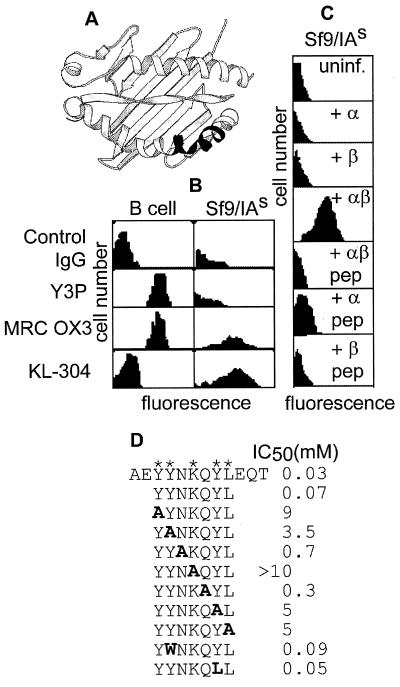
In studies of recombinant expression of class II MHC molecules in insect cells, we used the monoclonal antibody KL-304 (23), which had been raised to a peptide epitope (β58–69) found as part of a highly polymorphic region of the antigen-binding site of the murine class II MHC protein IAs (Fig. 1A). (In humans, this region is relatively conserved.) KL-304 recognizes a recombinant form of IAs produced in baculovirus-infected insect cells but does not bind to native IAs on the surface of B cells (Fig. 1B). This behavior is unusual, because other antibodies tested reacted preferentially with the native protein or did not discriminate (Fig. 1B; L.S. and L.J.S., unpublished results). Antibody binding required expression of both IAs α and β subunits but was abrogated by addition to the culture medium of a peptide PLP [139–151], known to bind specifically to IAs (32) (Fig. 1C). Insect cells are deficient in loading endogenous peptide onto heterologously expressed class II MHC proteins (19), and we suspected that KL-304 might preferentially react with MHC molecules that had not acquired peptide.
Figure 1.
KL-304 reactivity with IAs from human and insect cells. (A) The KL-304 epitope (23) (Lower Right, shaded) on a ribbon diagram of the class II MHC peptide-binding site (36). (B) Flow cytometry of IAs/d B cells (B-cell lymphoma LS 102.9) or IAs-expressing insect cells (Sf9/IAs), by using control and MHC-specific antibodies, as indicated (Left). KL-304 preferentially stains recombinant insect cells, as compared with B cells. Y3P, MRC OX3, and other antibodies tested (10–2.16, MRC OX6, 10–3.6.2, MK-S4; not shown) preferentially stain B cells or do not discriminate. (C) Flow cytometry by using KL-304 of insect cells expressing individual IAs subunits α, β, or both, with or without antigenic peptide PLP[139–151] treatment. Only the empty αβ complex binds KL-304. (D) KL-304 epitope mapping. Substitutions of the IAs sequence shown in bold, with asterisks indicating positions of key residues.
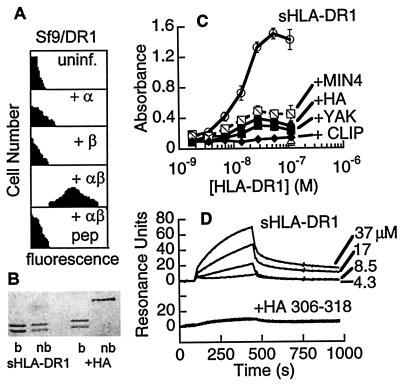
The human class II MHC molecule HLA-DR1 can be prepared in well-characterized empty and peptide-loaded forms (19, 20) and could provide a test of the hypothesis that KL-304 is specific for the empty MHC protein. Peptide-mapping experiments (Fig. 1D) show that the KL-304 epitope is discontinuous. The key epitope residues in IAs (YY-K-YL) are substantially conserved in HLA-DR1 (YW– – –K-LL), with the substitutions Y->W and Y->L not substantially affecting binding (Fig. 1D). HLA-DR1 has a two-residue insertion in the epitope region relative to IAs, but crystal structures show that the insertion forms a bulge without altering the helical register (36, 40, 41). KL-304 reacts with HLA-DR1 expressed in insect cells (Fig. 2A). As with IAs, KL-304 reactivity with HLA-DR1 required both α and β subunits and was abrogated by peptide binding (Fig. 2A).
Figure 2.
KL-304 specifically recognizes empty HLA-DR1. (A) Flow cytometry of insect cells expressing HLA-DR1, as in Fig. 1C, with or without HA peptide treatment. (B) SDS/PAGE of empty soluble HLA-DR1 produced in E. coli (sHLA-DR1) and the HLA-DR1-HA peptide complex (+HA), with samples boiled (+) or not (−) before loading. Subunit dissociation without boiling indicates the lack of associated peptides (19, 20). (C) Sandwich ELISA by using immobilized KL-304 to detect binding to empty soluble HLA-DR1 (open circles) and to various peptide complexes (MIN4, hatched squares; HA, solid circles; YAK, solid squares; CLIP, solid diamonds). Soluble HLA-DR1 showed no binding to heat-denatured KL-304 (x), nonspecific antibody (+), or BSA (open triangle). (D) Plasmon resonance by using immobilized KL-304 Fab, for empty HLA-DR1 (Top) or peptide-loaded HLA-DR1 (Bottom).
To establish that KL-304 reactivity was specific for empty MHC molecules and not for MHC complexes carrying peptides adventitiously derived from endogenous insect cell or serum proteins, we used recombinant soluble HLA-DR1 produced as individual subunits in E. coli and folded in vitro in the absence (Fig. 2B, lanes 1, 2) or presence (lanes 3, 4) of peptide (20). The folded peptide-free HLA-DR1 from E. coli showed strong KL-304 reactivity as assayed by sandwich ELISA (Fig. 2C) and plasmon resonance (Fig. 2D). We tested several peptide complexes of HLA-DR1 for binding to KL-304, including those of the tight-binding antigenic peptide HA (Kd ≈ 10−8 M), the polyalanine analogue YAK (Kd ≈ 10−7 M), the weakly binding minimal tetrameric peptide MIN4 (Kd ≈ 10−5 M), and the invariant chain fragment CLIP (Kd ≈ 10−8 M) (37). None of these peptide complexes exhibited significant binding to KL-304 (Fig. 2B). Thus, KL-304 appears to be specific for the empty MHC protein, most likely by sensing a conformational change that has been reported to occur in class II MHC proteins on peptide binding (37, 42, 43).
Empty MHC Molecules Are Expressed on the Surface of Immature DC.
We used KL-304 to investigate expression of empty class II MHC proteins on the surface of various professional antigen-presenting cells using primary cell cultures from IAs mice. Splenic B cells, peritoneal B cells, splenic macrophages, and peritoneal macrophages all expressed little or no empty class II MHC protein at the cell surface, as detected by KL-304 (Table 1, Fig. 3 D and E). All of these cells expressed substantial amounts of class II MHC-peptide complexes as detected by the IA-specific monoclonal antibodies 10–2.16, Y3P, and 11–5.2. In marked contrast, DC isolated from spleen or derived from bone-marrow precursors expressed high levels of empty cell-surface class II MHC, as detected by KL-304 (Table 1, Fig. 3 A–C). DC from mice carrying IAk, IAu, and IAf alleles, which contain the KL-304 epitope sequence, also were able to bind KL-304 (Table 1). DC from mice carrying noncrossreactive MHC alleles IAd and IAb or from class II knockout mice (30) did not react with KL-304. Thus, both splenic and bone-marrow derived DC, but not other antigen-presenting cells, react specifically with KL-304 and appear to express substantial amounts of empty cell-surface class II MHC molecules. We cannot exclude the possibility that B cells and macrophages express empty class II MHC molecules in a conformation not recognized by KL-304 or in a short-lived state not detected by our assays.
Table 1.
Empty and peptide-loaded cell-surface class II MHC molecules on antigen-presenting cells
| Cell type/Source | Haplotype | KL-304* | 10-2.16* | Y3P* | 11-5.2* | Control IG*† |
|---|---|---|---|---|---|---|
| B cells | ||||||
| Splenic | s | 2 (4) | 87 (21) | 86 (20) | — | 2 (4) |
| Splenic | k | 2 (4) | 98 (20) | — | 84 (21) | 3 (5) |
| Peritoneal | s | 2 (5) | 83 (22) | 82 (20) | — | 2 (1) |
| L.S.102.9‡ | s/d | 1 (6) | 90 (58) | 96 (45) | — | 2 (1) |
| Macrophages | ||||||
| Splenic | s | 8 (5) | 94 (24) | 91 (22) | — | 2 (2) |
| Splenic | k | 6 (4) | 92 (25) | — | 93 (26) | 3 (3) |
| Peritoneal | s | 5 (2) | 27 (28) | 24 (27) | — | 2 (2) |
| Peritoneal | k | 3 (2) | 35 (23) | — | 30 (25) | 3 (2) |
| Dendritic cells | ||||||
| Splenic | s | 93 (57) | 95 (330) | 94 (340) | — | 3 (4) |
| Splenic | k | 98 (89) | 97 (540) | — | 98 (771) | 3 (8) |
| Bone marrow | s | 90 (44) | 90 (58) | 90 (55) | — | 2 (3) |
| Bone marrow | k | 90 (36) | 59 (48) | — | 67 (45) | 2 (2) |
| Splenic | u | 89 (99) | 91 (279) | 93 (393) | — | 3 (8) |
| Splenic | f | 83 (45) | 83 (57) | 87 (84) | — | 2 (1) |
| Splenic | MHC II0/0 | 8 (4) | — | 7 (6) | — | 10 (5) |
Percentage of cells staining positively for the indicated antibody, with mean fluorescence intensity in parentheses.
Average values for isotype matched control antibodies.
B-cell lymphoma.
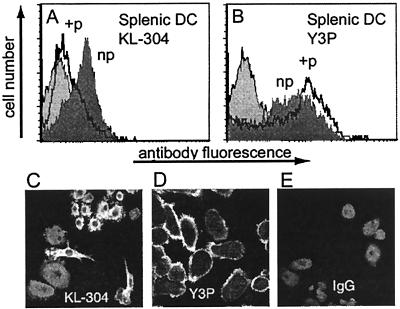
The fraction of the total cell-surface MHC in the empty KL-304-reactive form varied with the DC developmental state. Bone marrow-derived DC can be obtained in various developmental states depending on the time of culture and GM-CSF treatment (44). Higher expression levels of empty class II MHC proteins were observed for more immature DC obtained by culture in GM-CSF, and lower levels were observed for more mature DC, obtained by culture with TNFα, which induces DC maturation (Fig. 3 A–C). Y3P expression varied reciprocally with DC maturation state. Splenic DC, which comprise a mixture of intermediate developmental states, expressed an intermediate level of KL-304 reactive class II MHC molecules (Fig. 4A). Using the splenic DC, we confirmed that KL-304 reactivity was blocked by peptide binding, as observed for IAs from insect cells. After addition of antigenic peptide PLP [139–151] to the culture medium, the level of empty cell-surface MHC decreased (Fig. 4A), with a concomitant increase in level of the peptide-loaded form as detected with the complex-specific antibody Y3P (Fig. 4B). Nonbinding control peptide PLP [35–50] had no effect on KL-304 or Y3P surface expression (not shown). Confocal microscopy of splenic DC confirmed the developmental regulation of empty MHC class II expression. KL-304 reactivity to splenic DC (Fig. 4C) was associated with several immature and intermediate phenotypes (31, 44, 45), whereas Y3P reactivity (Fig. 4D) was mostly associated with the more mature sea urchin-like phenotype previously correlated with the high levels of class II MHC surface expression (31, 44, 45).
Figure 4.
Splenic DC express empty class II MHC inhibited by peptide binding (A and B) Flow cytometry of splenic DC either untreated (np, shaded) or preincubated with PLP [139–151] peptide (+p, unshaded) by using antibodies KL-304 (F) or Y3P (G). Control IgG staining shown with light shading. (C–E) Fluorescence microscopy of splenic DC labeled with KL-304 (C) Y3P (D), or control antibody (E).
Empty Cell Surface MHC Class II Proteins Are Functional in Antigen Binding.
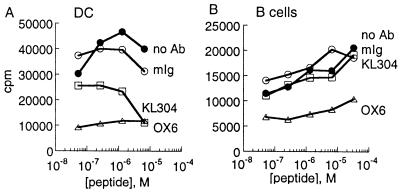
To investigate the functional capacity of the empty class II MHC expressed on the surface of DC, we performed peptide-binding experiments with a fluorescein-labeled version of the antigenic peptide PLP[139–151]. Cells were treated with sodium azide (12 mM) to prevent endocytosis and other energy-requiring processes, washed, and then incubated with peptide in a 2-hr binding assay. Immature DC efficiently bound added antigenic peptide PLP[139–151], whereas control peptide PLP[35–50] did not bind, as shown by flow cytometry (Fig. 5A). Cell-surface peptide binding varied with DC developmental state, with more immature DC exhibiting more peptide-binding activity (Fig. 5 A–C). Peptide-binding levels were low for B cells (Fig. 5D) and macrophages (Fig. 5E), consistent with previous studies showing inefficient cell-surface peptide binding to B cells (46, 47). In these experiments, B cells and macrophages were treated with (bacterial) lipopolysaccharide to up-regulate surface expression to levels comparable to those of the DC. Untreated B cells and macrophages also showed minimal peptide-binding activity (not shown). These results indicate that empty IAs on the surface of DC has retained its specific peptide-binding function.
We investigated the binding of antigenic peptides to other class II MHC allotypes in addition to IAs. Peptides restricted to IAb and IAk bound efficiently to DC but not B cells from mice carrying these MHC haplotypes (Fig. 5 F–H). In each case, peptide binding to DC was concentration dependent and specific for the appropriate peptide. For IAk, empty cell-surface MHC molecules had been detected by KL-304 (Table 1), and the peptide-binding results suggest that the empty IAk is functional in specific binding. For IAb, the sequence varies from that of IAs and IAk in the KL-304 epitope region (23), and the presence of empty molecules on the DC surface cannot be established by using KL-304 binding. The high level of peptide binding observed for IAb on DC relative to B cells suggests that IAb, as well as IAs and IAk, is present at least partially as empty molecules on the DC surface.
Empty Cell-Surface MHC Class II Molecules Are Functional in T-Cell Activation.
To test whether the empty class II molecules on the DC surface were functional in antigen presentation to T cells, we used a T-cell line specific for IAs bound to PLP [139–151] and measured T-cell proliferation in response to peptide-loaded IAs splenic DC and B cells. At all antigen concentrations tested, DC were more efficient in inducing T-cell proliferation than were B cells (Fig. 6, circles, note different scales). DC are particularly potent antigen-presenting cells, and their increased ability relative to B cells in stimulating T-cell proliferation in this assay likely is because of increased costimulatory capacity as well as increased peptide loading. To evaluate the contribution to T-cell activation of MHC-peptide complexes derived from the empty cell-surface IAs, we incubated the antigen-presenting cells during the T-cell assay with antibodies that bind empty IAs (KL-304) or both empty and peptide-loaded forms (MRC-OX6). Antibody KL-304, specific for empty IAs, reduced T-cell activation by DC but not by B cells (Fig. 6, squares). Antibody MRC-OX6, which binds both empty and peptide-loaded IA, substantially blocked T-cell activation by both DC and B cells (Fig. 6, triangles). An isotype-matched control antibody had no significant effect on blocking T-cell proliferation (Fig. 6, open circles). These results show that the empty KL-304-reactive cell-surface IAs molecules are able to present bound peptide antigen to T cells, and that they contribute substantially to the activity of DC in presenting peptides derived from the extracellular medium.
Figure 6.
Inhibition of DC-stimulated T-cell proliferation by KL-304 antigen-specific presentation to an IAs-restricted T-cell line by DC (A) or B cells (B) loaded with various concentrations of PLP[139–151], in the absence of antibody (open circles) or the presence of antibodies KL-304 (squares, specific for empty IAs),MRC OX6 (triangles, binds both empty and peptide-loaded IA), or nonspecific Y6P control IgG (circles). T-cell proliferation was measured by [3H]thymidine incorporation.
Discussion
An antibody specific for the peptide-free conformation of class II MHC molecules revealed that immature DC can express a substantial fraction of their cell-surface class II MHC molecules in an empty peptide-receptive state. The empty molecules were detected on splenic and bone-marrow-derived DC. They appear to be fully active in specific peptide binding and can take up antigen directly from the extracellular medium. Bound antigens can be presented to T cells. The presence of such empty MHC class II molecules on the surface of DC was unexpected, because previous work in B cells suggested that MHC class II molecules that do not acquire peptide are retained intracellularly and degraded (21, 48). However, we note that empty class II MHC proteins are stable at physiological temperature (37, 49), unlike empty class I MHC proteins (49–51), and that class II MHC proteins can be produced in an empty or functionally empty form in several insect and mammalian cell lines (19, 52, 53). Thus, the intracellular retention of empty molecules observed in B cells may be caused by B-cell-specific mechanism rather than an intrinsic instability of empty class II MHC proteins.
Mice deficient in the class II-associated Ii chaperone have revealed different requirements for surface expression of MHC class II molecules in DC and B cells. In the absence of invariant chain, IA αβ heterodimers assemble but do not associate with peptide in the endoplasmic reticulum (ER) (48), and are found in a floppy (54) or peptide-receptive (55) state. In B cells, the IA is retained in the ER, but in DC it is expressed at the cell surface (17). This Ii-independent pathway could provide a possible model for trafficking of empty molecules to the DC surface. Another possible source for the empty class II MHC could be nascent MHC-Ii complexes that traffic to the cell surface rather than directly to endosomes, in a pathway reported to be particularly prominent in DC (56). Some of these molecules could release Ii or its proteolytic fragments at the surface to become the empty molecules observed here. In this regard, it has been reported that DC can express significant levels of cell-surface DM (57, 58, 66), which could help catalyze the release of Ii.
The empty cell-surface class II MHC molecules observed here could function as antigen receptors for DC. If so, the developmental regulation of expression of the empty molecules would be consistent with the role of immature DC in collecting antigen for storage and subsequent presentation to T cells. Several mechanisms for a physiological role of the empty cell-surface class II MHC molecules can be envisioned. Similarly to previously identified DC endocytic receptors such as the mannose (59) and Fc (60) receptors and DEC-205 (27), empty class II MHC molecules might serve as antigen-specific endocytic receptors. Class II MHC proteins have been shown to bind large protein fragments and even intact proteins (61, 62) and could bind unfolded or partially digested proteins that might be present at sites of inflammation and cell lysis with subsequent endocytosis and transport to a proteolytic compartment for further processing. In this respect, the empty molecules could function in a surface recycling pathway, as previously described for peptide loaded class II. This recycling pathway is active in both B cells and DC, appears to be independent of Ii synthesis (63), and has been shown to be fully active in delivering antigenic protein to endocytic compartments and back to the surface (12).
In an alternative mechanism, empty cell-surface class II MHC molecules could bind peptide antigens at the cell surface. In this mode, empty MHC molecules could capture peptide antigens directly from the extracellular milieu and present them to T cells without internalization or further processing. Such peptides could be generated at sites of inflammation by the proteolytic activities of macrophages and granulocytes or released as a result of cell lysis. DC themselves have been reported to express extracellular proteolytic activity that could contribute to antigen processing (64, 65, 66). This pathway could be extremely useful to the sentinel function of DC and would serve to broaden the peptide repertoire by preserving antigenic peptides that might otherwise be terminally degraded in the extremely proteolytic endosomal/lysosomal environment.
Acknowledgments
We thank Jennifer Zarutskie for empty and peptide-loaded HLA-DR1 from E. coli, Daniel DeOliveira for peptide synthesis, Mei Ling Wong for cell culture and flow cytometry, other members of the Stern laboratory for experimental assistance, and Jack Strominger for helpful comments. This work was supported by grants from the National Institutes of Health to L.J.S. (RO1-AI38996) and M.E.D. (RO1-CA67416), and a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft to F.F.
Abbreviations
- Ii
invariant chain
- DC
dendritic cells
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- TNFα
tumor necrosis factor α
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Germain R N. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Watts C. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 3.Pieters J, Bakke O, Dobberstein B. J Cell Sci. 1993;106:831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 4.Benaroch P, Yilla M, Raposo G, Ito K, Miwa K, Geuze H J, Ploegh H L. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche P A, Teletski C L, Stang E, Bakke O, Long E O. Proc Natl Acad Sci USA. 1993;90:8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salamero J, Humbert M, Cosson P, Davoust J. EMBO J. 1990;9:3489–3496. doi: 10.1002/j.1460-2075.1990.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf P R, Ploegh H L. Nature (London) 1995;376:464–465. doi: 10.1038/376464a0. [DOI] [PubMed] [Google Scholar]
- 8.Riese R J, Mitchell R N, Villadangos J A, Shi G P, Palmer J T, Karp E R, De Sanctis G T, Ploegh H L, Chapman H A. J Clin Invest. 1998;101:2351–2363. doi: 10.1172/JCI1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denzin L K, Cresswell P. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 10.Germain R N, Hendrix L R. Nature (London) 1991;353:134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 11.German R N, Castellino F, Han R, Reis e Sousa C, Romagnoli P, Sadegh-Nasseri S, Zhong G M. Immunol Rev. 1996;151:5–30. doi: 10.1111/j.1600-065x.1996.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 12.Pinet V M, Long E O. Eur J Immunol. 1998;28:799–804. doi: 10.1002/(SICI)1521-4141(199803)28:03<799::AID-IMMU799>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Muhlethaler-Mottet A, Otten L A, Steimle V, Mach B. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C H, Guerder S, Hong S C, van Ewijk W, Flavell R A. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 15.Williams G S, Malin M, Vremec D, Chang C H, Boyd R, Benoist C, Mathis D. Int Immunol. 1998;10:1957–1967. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- 16.Clausen B E, Waldburger J M, Schwenk F, Barras E, Mach B, Rajewsky K, Forster I, Reith W. Immunity. 1998;8:143–155. doi: 10.1016/s1074-7613(00)80467-7. [DOI] [PubMed] [Google Scholar]
- 17.Rovere P, Zimmermann V S, Forquet F, Demandolx D, Trucy J, Ricciardi-Castagnoli P, Davoust J. Proc Natl Acad Sci USA. 1998;95:1067–1072. doi: 10.1073/pnas.95.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz M B, Rovere P, Kleijmeer M J, Assmann C U, Oorschot V M, Rescigno M, Geuze H J, Davoust J, Ricciardi-Castagnoli P. Adv Exp Med Biol. 1997;417:167–169. doi: 10.1007/978-1-4757-9966-8_27. [DOI] [PubMed] [Google Scholar]
- 19.Stern L J, Wiley D C. Cell. 1992;68:465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- 20.Frayser M, Sato A K, Xu L, Stern L J. Protein Expr Purif. 1999;15:105–114. doi: 10.1006/prep.1998.0987. [DOI] [PubMed] [Google Scholar]
- 21.Germain R N, Rinker A G., Jr Nature (London) 1993;363:725–728. doi: 10.1038/363725a0. [DOI] [PubMed] [Google Scholar]
- 22.Oi V T, Jones P P, Goding J W, Herzenberg L A. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- 23.LaPan K E, Klapper D G, Frelinger J A. Hybridoma. 1992;11:217–223. doi: 10.1089/hyb.1992.11.217. [DOI] [PubMed] [Google Scholar]
- 24.Pierres M, Marchetto S, Naquet P, Landais D, Peccoud J, Benoist C, Mathis D. J Exp Med. 1989;169:1655–1668. doi: 10.1084/jem.169.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappler J W, Skidmore B, White J, Marrack P. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, Swiggard W J, Heufler C, Peng M, Mirza A, Steinman R M, Nussenzweig M C. Nature (London) 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 28.Coligan J E. Current Protocols in Immunology. New York: Greene and Wiley; 1992. [Google Scholar]
- 29.Fukumoto T, McMaster W R, Williams A F. Eur J Immunol. 1982;12:237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 31.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer J M, Sobel R A, Sette A, Southwood S, Lees M B, Kuchroo V K. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 33.Wall K A, Hu J Y, Currier P, Southwood S, Sette A, Infante A J. J Immunol. 1994;152:4526–4536. [PubMed] [Google Scholar]
- 34.Zamvil S S, Mitchell D J, Moore A C, Schwarz A J, Stiefel W, Nelson P A, Rothbard J B, Steinman L. J Immunol. 1987;139:1075–1079. [PubMed] [Google Scholar]
- 35.Allen P M, Strydom D J, Unanue E R. Proc Natl Acad Sci USA. 1984;81:2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern L J, Brown J H, Jardetzky T S, Gorga J C, Urban R G, Strominger J L, Wiley D C. Nature (London) 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 37.Zarutskie J A, Sato A K, Rushe M M, Chan I C, Lomakin A, Benedek G B, Stern L J. Biochemistry. 1999;38:5878–5887. doi: 10.1021/bi983048m. [DOI] [PubMed] [Google Scholar]
- 38.Chicz R M, Urban R G, Lane W S, Gorga J C, Stern L J, Vignali D A, Strominger J L. Nature (London) 1992;358:764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 39.Santambrogio L, Lees M B, Sobel R A. J Neuroimmunol. 1998;81:1–13. doi: 10.1016/s0165-5728(97)00138-0. [DOI] [PubMed] [Google Scholar]
- 40.Scott C A, Peterson P A, Teyton L, Wilson I A. Immunity. 1998;8:319–329. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]
- 41.Fremont D H, Monnaie D, Nelson C A, Hendrickson W A, Unanue E R. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 42.Reich Z, Boniface J J, Lyons D S, Borochov N, Wachtel E J, Davis M M. Nature (London) 1997;387:617–620. doi: 10.1038/42500. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowitz J D, Vrljic M, Kasson P M, Liang M N, Busch R, Boniface J J, Davis M M, McConnell H M. Immunity. 1998;9:699–709. doi: 10.1016/s1074-7613(00)80667-6. [DOI] [PubMed] [Google Scholar]
- 44.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature (London) 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 45.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature (London) 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 46.Sherman M A, Weber D A, Spotts E A, Moore J C, Jensen P E. Cell Immunol. 1997;182:1–11. doi: 10.1006/cimm.1997.1219. [DOI] [PubMed] [Google Scholar]
- 47.Busch R, Rothbard J B. J Immunol Methods. 1990;134:1–22. doi: 10.1016/0022-1759(90)90107-7. [DOI] [PubMed] [Google Scholar]
- 48.Zhong G, Castellino F, Romagnoli P, Germain R N. J Exp Med. 1996;184:2061–2066. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reich Z, Altman J D, Boniface J J, Lyons D S, Kozono H, Ogg G, Morgan C, Davis M M. Proc Natl Acad Sci USA. 1997;94:2495–2500. doi: 10.1073/pnas.94.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahnestock M L, Tamir I, Narhi L, Bjorkman P J. Science. 1992;258:1658–1662. doi: 10.1126/science.1360705. [DOI] [PubMed] [Google Scholar]
- 51.Bouvier M, Wiley D C. Nat Struct Biol. 1998;5:377–384. doi: 10.1038/nsb0598-377. [DOI] [PubMed] [Google Scholar]
- 52.Jackson M R, Song E S, Yang Y, Peterson P A. Proc Natl Acad Sci USA. 1992;89:12117–12121. doi: 10.1073/pnas.89.24.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wettstein D A, Boniface J J, Reay P A, Schild H, Davis M M. J Exp Med. 1991;174:219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bikoff E K, Huang L Y, Episkopou V, van Meerwijk J, Germain R N, Robertson E J. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 56.Saudrais C, Spehner D, de la Salle H, Bohbot A, Cazenave J P, Goud B, Hanau D, Salamero J. J Immunol. 1998;160:2597–2607. [PubMed] [Google Scholar]
- 57.Kim K-J, Jung H-H, Bae Y S. Mol Cells. 1996;6:684–691. [Google Scholar]
- 58.Andersson T, Patwardhan A, Emilson A, Carlsson K, Scheynius A. Arch Dermatol Res. 1998;290:674–680. doi: 10.1007/s004030050372. [DOI] [PubMed] [Google Scholar]
- 59.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sette A, Adorini L, Colon S M, Buus S, Grey H M. J Immunol. 1989;143:1265–1267. [PubMed] [Google Scholar]
- 62.Castellino F, Zappacosta F, Coligan J E, Germain R N. J Immunol. 1998;161:4048–4057. [PubMed] [Google Scholar]
- 63.Zhong G, Romagnoli P, Germain R N. J Exp Med. 1997;185:429–438. doi: 10.1084/jem.185.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krahenbuhl O, Gattesco S, Tschopp J. Immunobiology. 1992;184:392–401. doi: 10.1016/S0171-2985(11)80596-6. [DOI] [PubMed] [Google Scholar]
- 65.Amoscato A A, Prenovitz D A, Lotze M T. J Immunol. 1998;161:4023–4032. [PubMed] [Google Scholar]
- 66.Santambrogiu L, Sato A K, Carver G J, Belyanskaya S L, Strominger J L, Stern L J. Proc Natl Acad Sci USA. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]