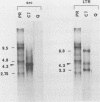
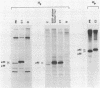
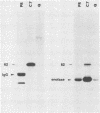
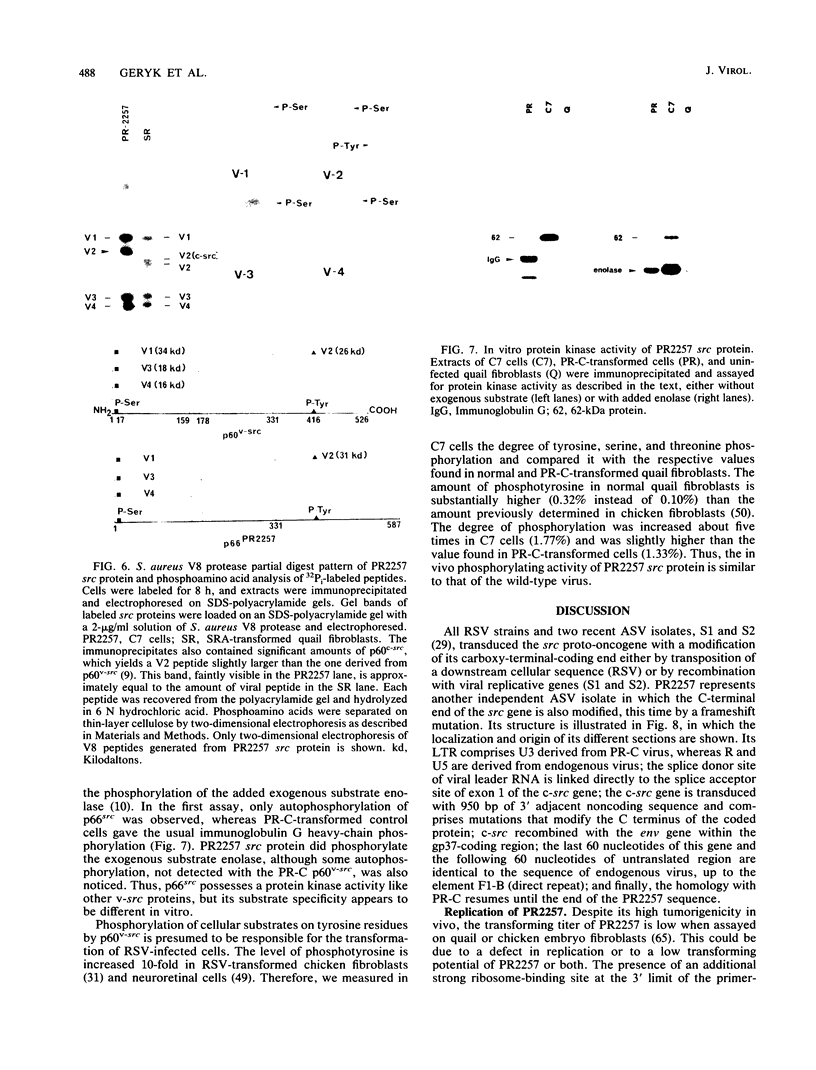
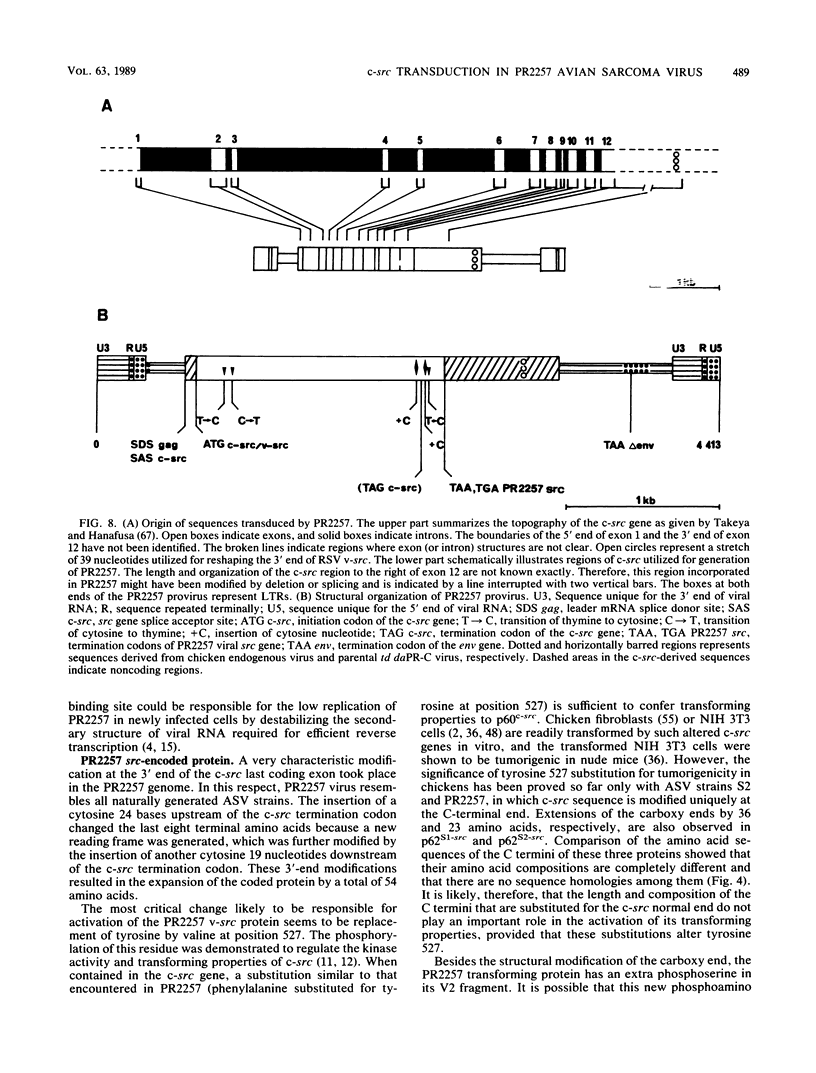
Abstract
When injected into chickens, a transformation-defective mutant of the Prague C strain of Rous sarcoma virus induced tumors at low incidence and after a long latency. One such tumor released a replication-defective virus designated PR2257. We molecularly cloned and sequenced the proviral DNA from quail fibroblasts transformed by PR2257. Comparison of PR2257 sequence with that of Prague C, cellular src, and 3' adjacent cellular DNA showed that the spliced version of the c-src gene and about 950 base pairs (bp) of 3'-flanking cellular DNA were transduced into PR2257. This transduction eliminated nearly all replicative genes, since the gag gene splice donor site was linked to the splice acceptor site of the src gene and, on the 3' side, recombination occurred in the end of env gene. Insertion of two extra cytosines 23 bp before and 19 bp after the c-src stop codon resulted in an extension of the coding portion up to 587 amino acids, divergence of sequences after Pro-525 and replacement of Tyr-527 by a valine residue. In addition, it appears that the 5' and 3' untranslated regions of PR2257 result from multiple recombinations between exogenous and endogenous virus genomes. Limited digestion of p66src encoded by PR2257 with Staphylococcus aureus V8 protease yielded a V2 peptide (C-terminal moiety) with an apparent molecular mass of 31 kilodaltons, consistent with the 5.7-kilodalton increase expected from the DNA sequence. The structure of PR2257 suggests that the first step in the capture of c-src gene by avian lymphomatosis viruses is the trans splicing of the viral leader mRNA to exon 1 of c-src.
Full text
PDF

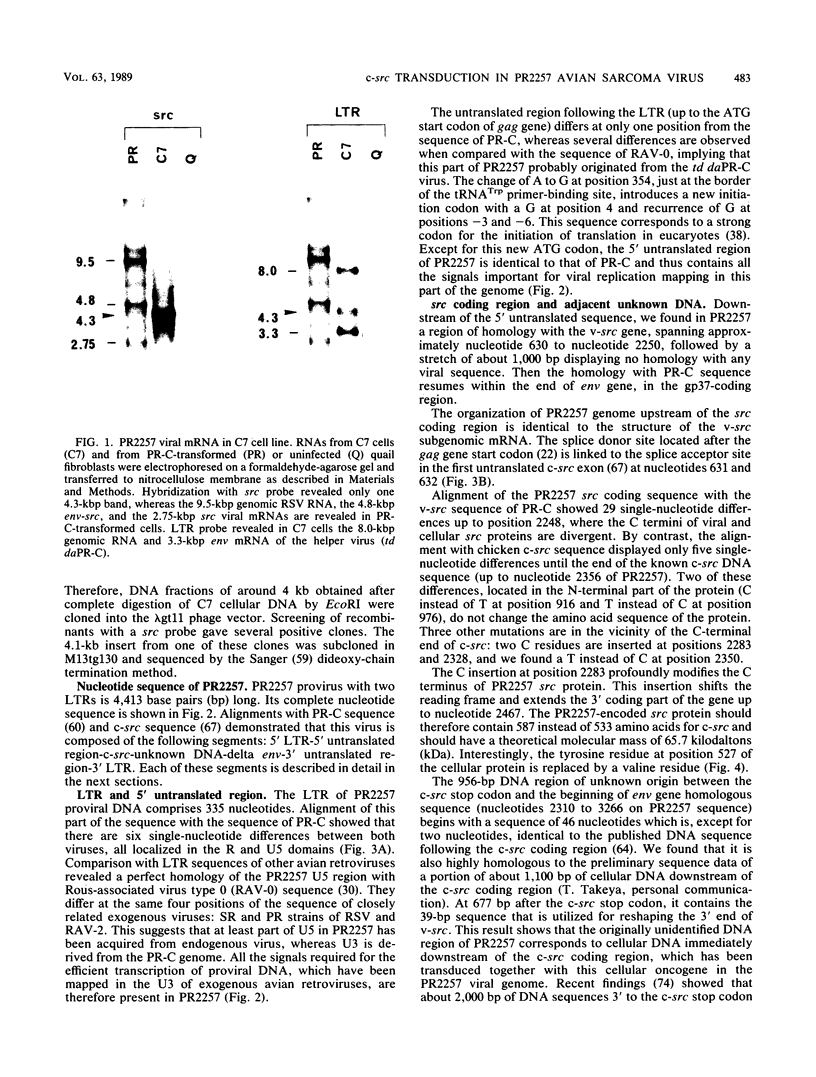







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggs P. M., Milne B. S., Graf T., Bauer H. Oncogenicity of non-transforming mutants of avian sarcoma viruses. J Gen Virol. 1973 Mar;18(3):399–403. doi: 10.1099/0022-1317-18-3-399. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Soskey L., Leis J. A retroviral RNA secondary structure required for efficient initiation of reverse transcription. J Virol. 1988 Oct;62(10):3622–3630. doi: 10.1128/jvi.62.10.3622-3630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Transcriptional activity of avian retroviral long terminal repeats directly correlates with enhancer activity. J Virol. 1985 Feb;53(2):515–521. doi: 10.1128/jvi.53.2.515-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., DeLorbe W., Swanstrom R., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. The nucleotide sequence of an untranslated but conserved domain at the 3' end of the avian sarcoma virus genome. Nucleic Acids Res. 1980 Jul 11;8(13):2967–2984. doi: 10.1093/nar/8.13.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L. Circularization of retroviral genomic RNA and the control of RNA translation, packaging and reverse transcription. Biochimie. 1986 Jul-Aug;68(7-8):941–949. doi: 10.1016/s0300-9084(86)81057-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A., Jaton J. C. In vitro, the major ribosome binding site on Rous sarcoma virus RNA does not contain the nucleotide sequence coding for the N-terminal amino acids of the gag gene product. J Virol. 1979 Feb;29(2):597–611. doi: 10.1128/jvi.29.2.597-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Parsons J. T. Identification of transcriptional elements within the long terminal repeat of Rous sarcoma virus. Mol Cell Biol. 1983 Oct;3(10):1834–1845. doi: 10.1128/mcb.3.10.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gudkov A. V., Korec E., Chernov M. V., Tikhonenko A. T., Obukh I. B., Hlozánek I. Genetic structure of the endogenous proviruses and expression of the gag gene in Brown Leghorn chickens. Folia Biol (Praha) 1986;32(1):65–72. [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Varmus H. E., Bishop J. M. The genesis of Rous sarcoma virus messenger RNAs. Virology. 1981 Jul 30;112(2):714–728. doi: 10.1016/0042-6822(81)90316-0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Differential transcription from the long terminal repeats of integrated avian leukosis virus DNA. J Virol. 1986 Nov;60(2):497–505. doi: 10.1128/jvi.60.2.497-505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara H., Shimizu T., Yamamoto H., Yoshino T. Two strains of avian sarcoma virus newly isolated from chick fibrosarcomas induced by lymphatic leukemia virus subgroup A in two lines of chickens. J Natl Cancer Inst. 1984 Mar;72(3):631–635. [PubMed] [Google Scholar]
- Hughes S. H. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus 0. J Virol. 1982 Jul;43(1):191–200. doi: 10.1128/jvi.43.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Hagino-Yamagishi K., Kawai S., Yamamoto T., Toyoshima K. Activation of the cellular src gene by transducing retrovirus. Mol Cell Biol. 1986 Jul;6(7):2420–2428. doi: 10.1128/mcb.6.7.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987 Apr 10;49(1):65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Trans splicing of mRNA precursors in vitro. Cell. 1985 Aug;42(1):165–171. doi: 10.1016/s0092-8674(85)80112-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugier D., Marx M., Barnier J. V., Poirier F., Genvrin P., Dezélée P., Calothy G. N-terminal deletion in the src gene of Rous sarcoma virus results in synthesis of a 45,000-Mr protein with mitogenic activity. J Virol. 1987 Aug;61(8):2523–2529. doi: 10.1128/jvi.61.8.2523-2529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lerner T. L., Hanafusa H. DNA sequence of the Bryan high-titer strain of Rous sarcoma virus: extent of env deletion and possible genealogical relationship with other viral strains. J Virol. 1984 Feb;49(2):549–556. doi: 10.1128/jvi.49.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Levine A. J. The group C adenovirus tumor antigens: identification in infected and transformed cells and a peptide map analysis. Cell. 1977 Aug;11(4):871–879. doi: 10.1016/0092-8674(77)90298-7. [DOI] [PubMed] [Google Scholar]
- Marx M., Crisanti P., Eychène A., Béchade C., Laugier D., Ghysdaël J., Pessac B., Calothy G. Activation and transduction of c-mil sequences in chicken neuroretina cells induced to proliferate by infection with avian lymphomatosis virus. J Virol. 1988 Dec;62(12):4627–4633. doi: 10.1128/jvi.62.12.4627-4633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Wang L. H. Mechanisms for the generation of src-deletion mutants and recovered sarcoma viruses: identification of viral sequences involved in src deletions and in recombination with c-src sequences. Virology. 1984 Oct 30;138(2):236–245. doi: 10.1016/0042-6822(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Poirier F., Calothy G., Karess R. E., Erikson E., Hanafusa H. Role of p60src kinase activity in the induction of neuroretinal cell proliferation by rous sarcoma virus. J Virol. 1982 Jun;42(3):780–789. doi: 10.1128/jvi.42.3.780-789.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier F., Jullien P., Dezelee P., Dambrine G., Esnault E., Benatre A., Calothy G. Role of the mitogenic property and kinase activity of p60src in tumor formation by Rous sarcoma virus. J Virol. 1984 Feb;49(2):325–332. doi: 10.1128/jvi.49.2.325-332.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamer R. H., Okazaki W. Evidence for the defectiveness of the Harris strain of Rous sarcoma virus. J Natl Cancer Inst. 1970 Apr;44(4):763–767. [PubMed] [Google Scholar]
- Reynolds A. B., Vila J., Lansing T. J., Potts W. M., Weber M. J., Parsons J. T. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987 Aug;6(8):2359–2364. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Trans splicing: variation on a familiar theme? Cell. 1987 Jul 17;50(2):147–148. doi: 10.1016/0092-8674(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Dvorák M., Guntaka R., Geryk J. Transmission of (LTR, v-src, LTR) without recombination with a helper virus. Virology. 1986 Sep;153(2):314–317. doi: 10.1016/0042-6822(86)90035-8. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Geryk J., Karakoz I., Rejthar A. Isolation of two transforming viruses from sarcomas obtained in chickens inoculated intraembryonally with a transformation defective mutant of Prague strain Rous sarcoma virus. Folia Biol (Praha) 1985;31(2):135–151. [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Donehower L., Hager G., Zeller N., Malavarca R., Astrin S., Skalka A. M. Sequence comparison in the crossover region of an oncogenic avian retrovirus recombinant and its nononcogenic parent: genetic regions that control growth rate and oncogenic potential. Mol Cell Biol. 1982 Nov;2(11):1331–1338. doi: 10.1128/mcb.2.11.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Beckson M., Anderson S. M., Hanafusa H. Identification of the viral sequence required for the generation of recovered avian sarcoma viruses and characterization of a series of replication-defective recovered avian sarcoma viruses. J Virol. 1984 Mar;49(3):881–891. doi: 10.1128/jvi.49.3.881-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Iijima S., Dorai T., Lin B. Regulation of the expression of proto-oncogene c-src by alternative RNA splicing in chicken skeletal muscle. Oncogene Res. 1987 Jun;1(1):43–59. [PubMed] [Google Scholar]
- Yaciuk P., Shalloway D. Features of the pp60v-src carboxyl terminus that are required for transformation. Mol Cell Biol. 1986 Aug;6(8):2807–2819. doi: 10.1128/mcb.6.8.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]