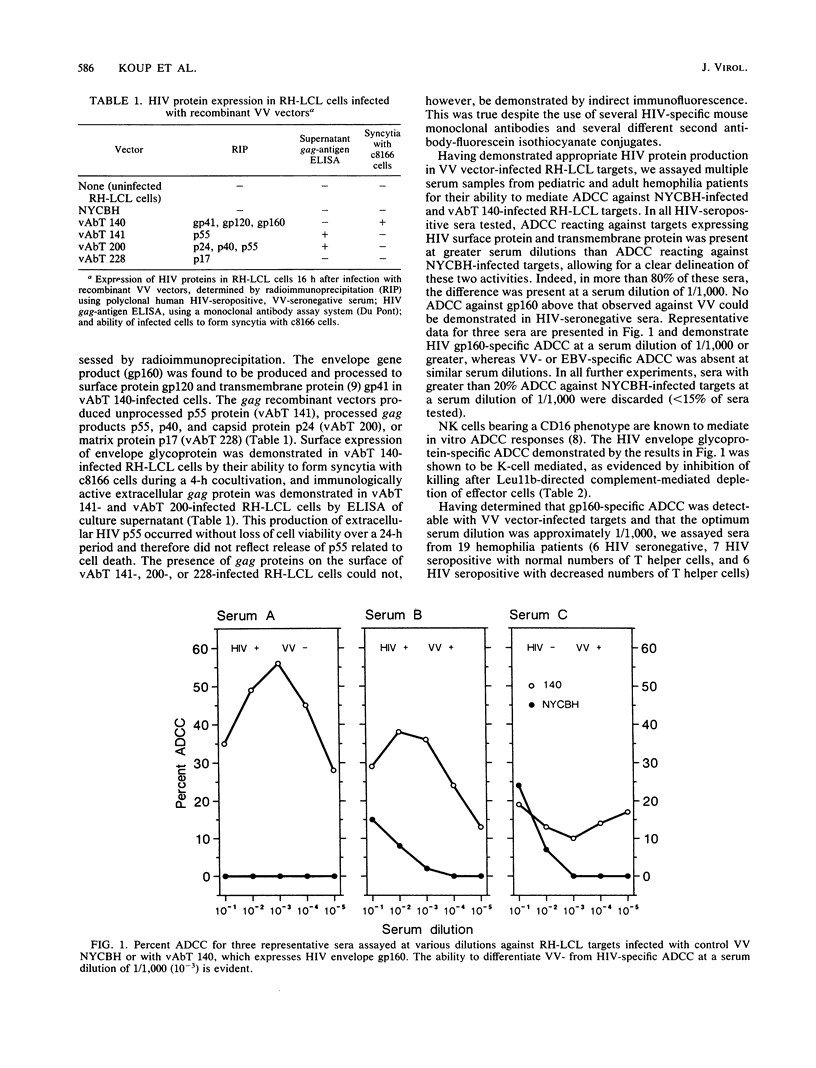
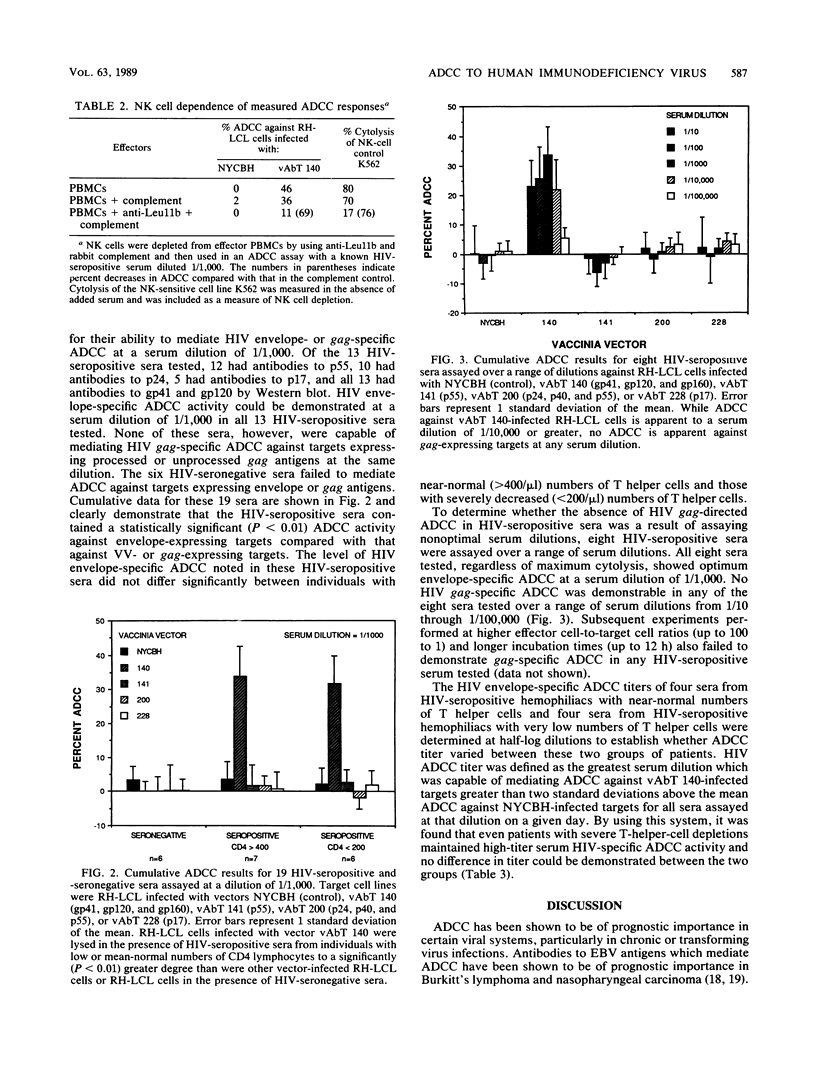
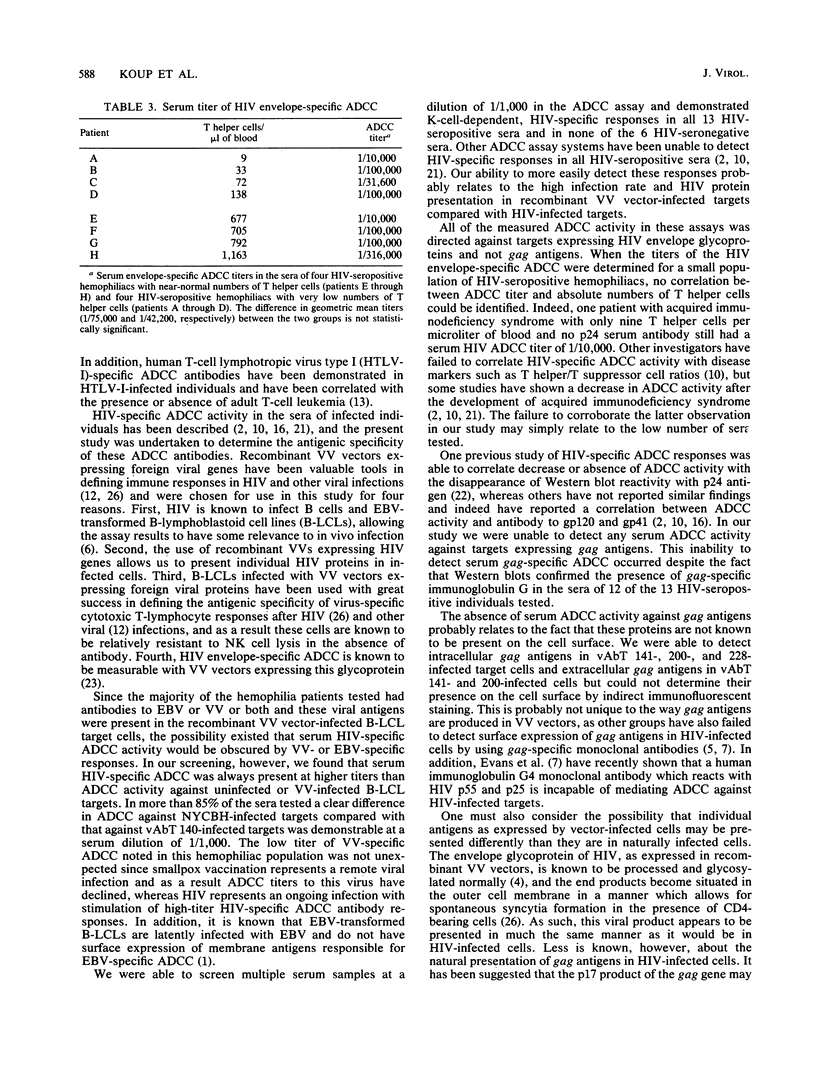
Abstract
Antibody-dependent cell-mediated cytotoxicity (ADCC) specific for human immunodeficiency virus (HIV) has been described for HIV-infected individuals. To determine the antigenic specificity of this immune response and to define its relationship to the disease state, an ADCC assay was developed using Epstein-Barr virus-transformed lymphoblastoid cell line targets infected with vaccinia virus vectors expressing HIV proteins. The vaccinia virus vectors induced appropriate HIV proteins (envelope glycoproteins gp160, gp120, and gp41 or gag proteins p55, p40, p24, and p17) in infected lymphoblastoid cell lines as demonstrated by radioimmunoprecipitation and syncytia formation with c8166 cells. Killer cell-mediated, HIV-specific ADCC was found in sera from HIV-seropositive but not HIV-seronegative hemophiliacs. This HIV-specific response was directed against envelope glycoprotein but was completely absent against target cells expressing the HIV gag proteins. The ADCC directed against gp160 was present at serum dilutions up to 1/316,000. There was no correlation between serum ADCC titer and the stage of HIV-related illness as determined by T-helper-cell numbers. These experiments clearly implicated gp160 as the target antigen of HIV-specific ADCC activity following natural infection. Vaccines which stimulate antibodies directed against gp160, which are capable of mediating ADCC against infected cells, could be important for protection against infection by cell-associated virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aya T., Mizuno F., Osato T. Immunologic cytotoxicity against autologous human lymphocytes transformed or infected by Epstein-Bar virus: role of antibody-dependent cellular cytotoxicity in health individuals. J Natl Cancer Inst. 1980 Aug;65(2):265–271. [PubMed] [Google Scholar]
- Brockman W. W., Nathans D. The isolation of simian virus 40 variants with specifically altered genomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):942–946. doi: 10.1073/pnas.71.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Robert-Guroff M., Wong-Staal F., Gallo R. C., Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986 Apr 10;320(6062):535–537. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- Chassagne J., Verrelle P., Dionet C., Clavel F., Barre-Sinoussi F., Chermann J. C., Montagnier L., Gluckman J. C., Klatzmann D. A monoclonal antibody against LAV gag precursor: use for viral protein analysis and antigenic expression in infected cells. J Immunol. 1986 Feb 15;136(4):1442–1445. [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Homsy J. M., Morrow W. J., Gaston I., Sooy C. D., Levy J. A. Human monoclonal antibody directed against gag gene products of the human immunodeficiency virus. J Immunol. 1988 Feb 1;140(3):941–943. [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren K., Böttiger B., Biberfeld G., Karlson A., Fenyö E. M., Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus. Presence at different clinical stages. J Immunol. 1987 Oct 1;139(7):2263–2267. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Miyakoshi H., Koide H., Aoki T. In vitro antibody-dependent cellular cytotoxicity against human T-cell leukemia/lymphoma virus (HTLV)-producing cells. Int J Cancer. 1984 Mar 15;33(3):287–291. doi: 10.1002/ijc.2910330302. [DOI] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Jaffe H. W., Spira T. J., Kennedy M. S., Jones B. M., Darrow W. W., Morgan M., Hubbard M. Exposure to human T-lymphotropic virus type III/lymphadenopathy-associated virus and immunologic abnormalities in asymptomatic homosexual men. Ann Intern Med. 1985 Jul;103(1):37–42. doi: 10.7326/0003-4819-103-1-37. [DOI] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Nishanian P., Keith D. E., Jr, Houghton R. L., Heitjan D. F., Fahey J. L., Giorgi J. V. Antibodies to human immunodeficiency virus in human sera induce cell-mediated lysis of human immunodeficiency virus-infected cells. J Immunol. 1987 Oct 1;139(7):2458–2463. [PubMed] [Google Scholar]
- Panicali D., Grzelecki A., Huang C. Vaccinia virus vectors utilizing the beta-galactosidase assay for rapid selection of recombinant viruses and measurement of gene expression. Gene. 1986;47(2-3):193–199. doi: 10.1016/0378-1119(86)90063-6. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Johansson B., Klein G. Antibody-dependent cellular cytotoxicity against Epstein-Barr virus-associated antigens in African patients with nasopharyngeal carcinoma. Int J Cancer. 1978 Aug 15;22(2):120–125. doi: 10.1002/ijc.2910220203. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Qualtiere L. F., Klein G., Norin T., Bal I. S. Epstein-Barr virus-specific antibody-dependent cellular cytotoxicity in patients with Burkitt's lymphoma. Int J Cancer. 1979 Oct 15;24(4):402–406. doi: 10.1002/ijc.2910240405. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Sarin P. S., Sun D. K., Thornton A. H., Naylor P. H., Goldstein A. L. Neutralization of HTLV-III/LAV replication by antiserum to thymosin alpha 1. Science. 1986 May 30;232(4754):1135–1137. doi: 10.1126/science.3010464. [DOI] [PubMed] [Google Scholar]
- Shepp D. H., Chakrabarti S., Moss B., Quinnan G. V., Jr Antibody-dependent cellular cytotoxicity specific for the envelope antigens of human immunodeficiency virus. J Infect Dis. 1988 Jun;157(6):1260–1264. doi: 10.1093/infdis/157.6.1260. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenqvist K., Sandberg T., Lidin-Janson G., Orskov F., Orskov I., Svanborg-Edén C. Virulence factors of Escherichia coli in urinary isolates from pregnant women. J Infect Dis. 1987 Dec;156(6):870–877. doi: 10.1093/infdis/156.6.870. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Brewster F. E., Brettler D. B., Forsberg A. D., Cheeseman S. H., Byron K. S., Baker S. M., Willitts D. L., Lew R. A., Levine P. H. Hemophiliac immunodeficiency: influence of exposure to factor VIII concentrate, LAV/HTLV-III, and herpesviruses. J Pediatr. 1986 Apr;108(4):504–510. doi: 10.1016/s0022-3476(86)80823-x. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Hänggi M., Hiller G. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J Virol. 1984 Feb;49(2):371–378. doi: 10.1128/jvi.49.2.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff H., Anderson D. J. Potential human immunodeficiency virus-host cells in human semen. AIDS Res Hum Retroviruses. 1988 Feb;4(1):1–2. doi: 10.1089/aid.1988.4.1. [DOI] [PubMed] [Google Scholar]


