Abstract
A stable temperature-sensitive mutant of measles virus (MV ts38) was used to study the mechanism of virus-mediated immune suppression of peripheral blood mononuclear cells in vitro. Both unstimulated and phytohemagglutinin-stimulated cultures released infectious virus at 32 degrees C, whereas no virus was released at 37 degrees C, although both viral RNA and viral proteins were synthesized. However, the response of the lymphoid cells to phytohemagglutinin, concanavalin A, and herpes simplex virus antigen was decreased in the presence of MV ts38 at 37 degrees C. The viability of infected cells was not diminished, therefore excluding cell death as a reason for immunosuppression. Interleukin 2 did not play a role in the inhibitory effect of MV ts38. Antibodies to alpha interferon partially reversed the inhibitory effect of the virus infection on lymphocyte mitogenesis, thus implying that alpha interferon plays a role in the immunosuppression. Depletion experiments indicated that adherent cells play a greater role in the measles virus-induced immunosuppression than nonadherent cells. However, monocyte maturation to macrophages had no effect on the degree of immunosuppression.
Full text
PDF

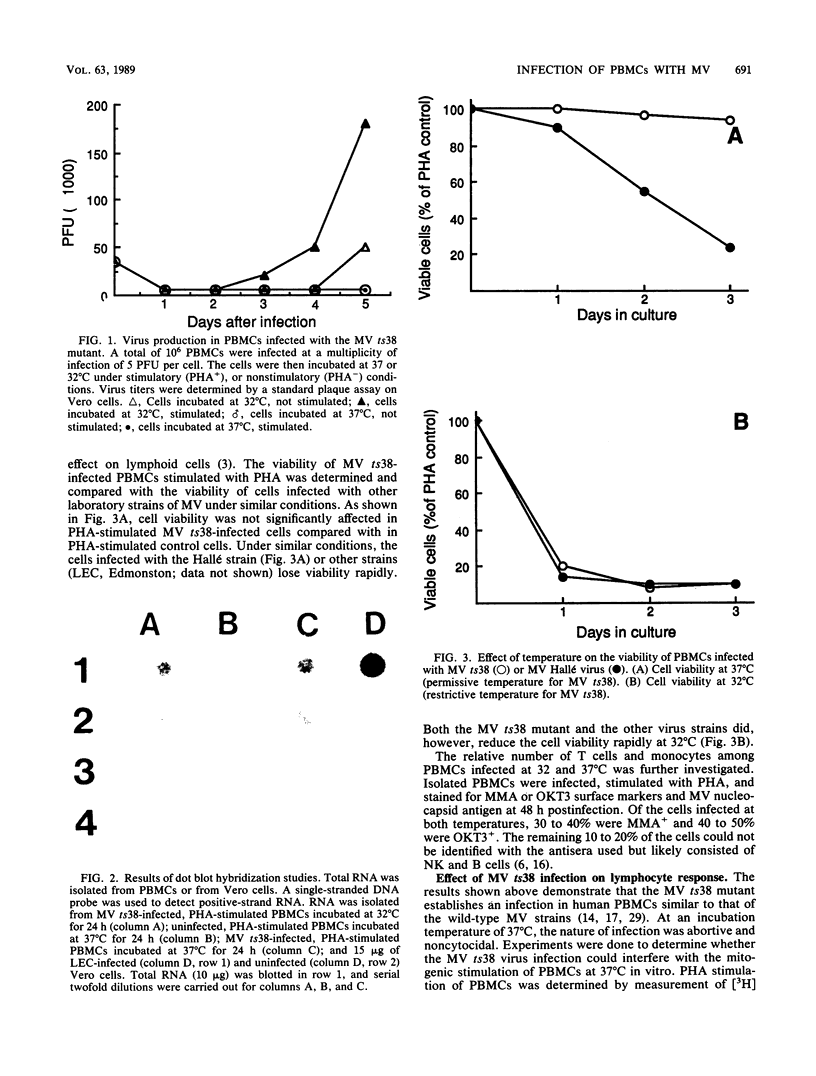
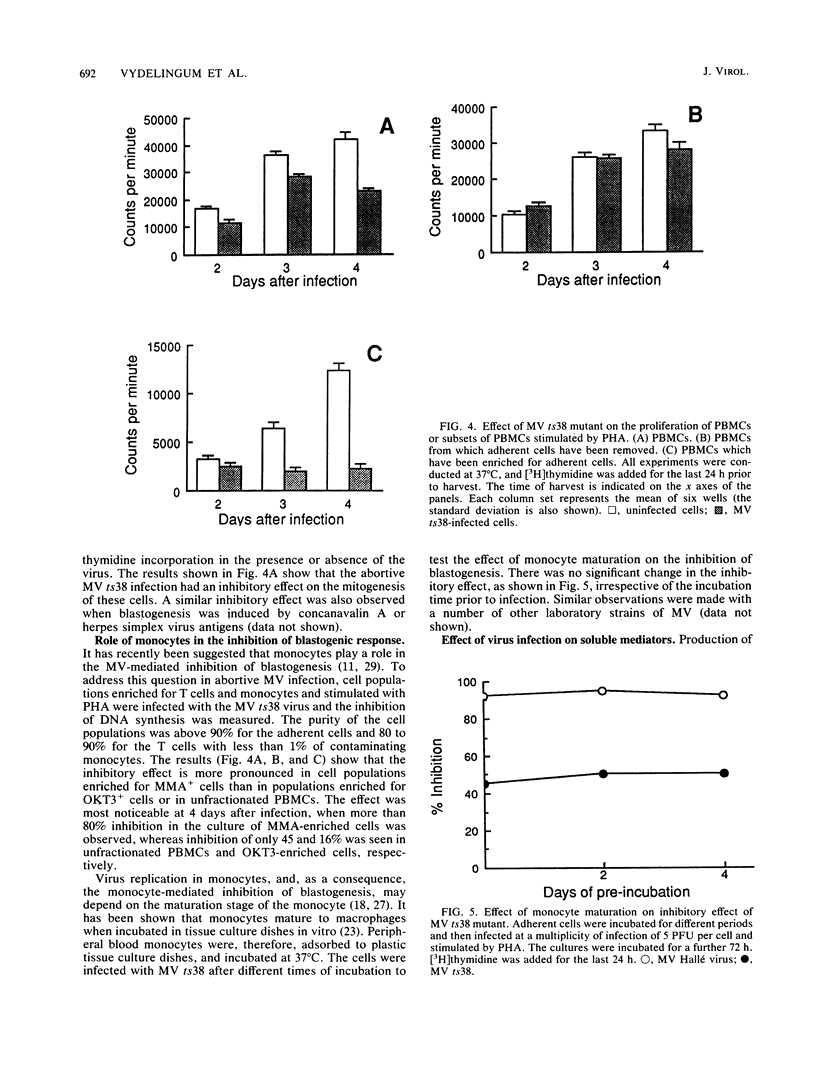
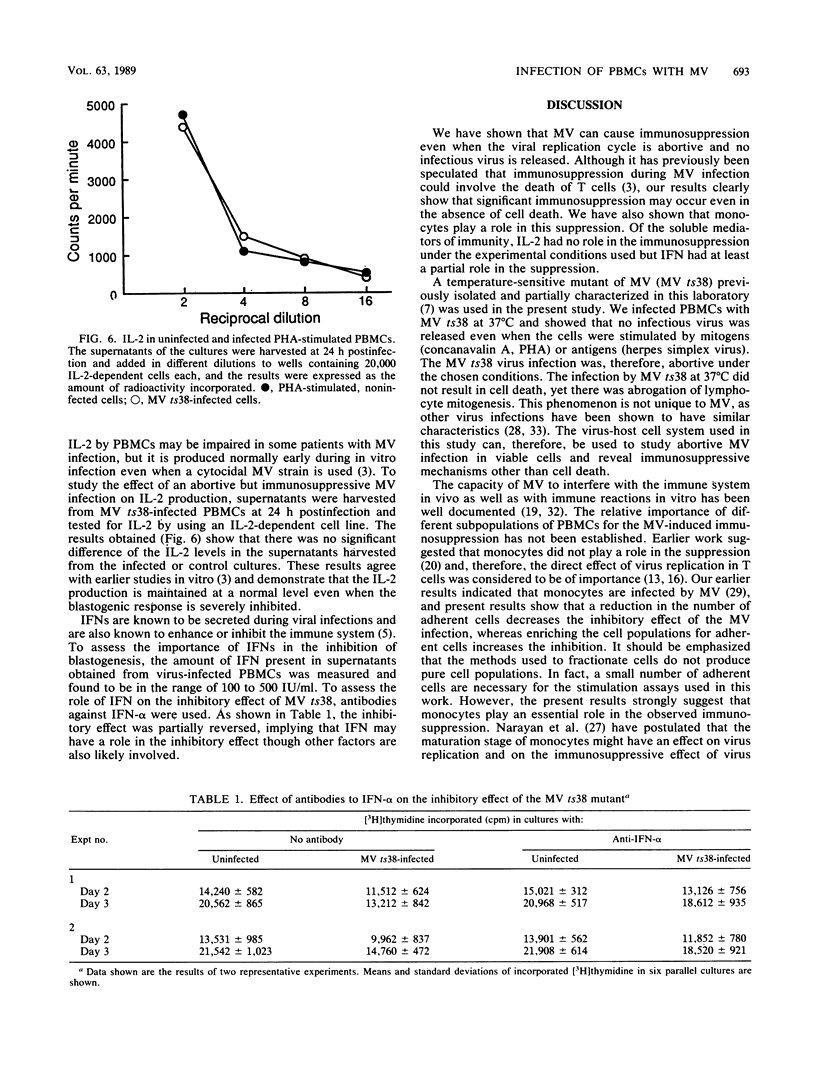


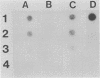
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basle M. F., Russell W. C., Goswami K. K., Rebel A., Giraudon P., Wild F., Filmon R. Paramyxovirus antigens in osteoclasts from Paget's bone tissue detected by monoclonal antibodies. J Gen Virol. 1985 Oct;66(Pt 10):2103–2110. doi: 10.1099/0022-1317-66-10-2103. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Casali P., Rogers B., Morris S., Sissons J. G. The immunosuppressive effects of measles virus on T cell function--failure to affect IL-2 release or cytotoxic T cell activity in vitro. Clin Exp Immunol. 1985 Jan;59(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Capobianchi M. R., Facchini J., Di Marco P., Antonelli G., Dianzani F. Induction of alpha interferon by membrane interaction between viral surface and peripheral blood mononuclear cells. Proc Soc Exp Biol Med. 1985 Apr;178(4):551–556. doi: 10.3181/00379727-178-42041. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui L. W., Vainionpä R., Marusyk R., Salmi A., Norrby E. Nuclear accumulation of measles virus nucleoprotein associated with a temperature-sensitive mutant. J Gen Virol. 1986 Oct;67(Pt 10):2153–2161. doi: 10.1099/0022-1317-67-10-2153. [DOI] [PubMed] [Google Scholar]
- Fireman P., Friday G., Kumate J. Effect of measles vaccine on immunologic responsiveness. Pediatrics. 1969 Feb;43(2):264–272. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glickman J. N., Howe J. G., Steitz J. A. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus-infected cells. J Virol. 1988 Mar;62(3):902–911. doi: 10.1128/jvi.62.3.902-911.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T., Tamashiro V. G., Moench T. R., Jauregui E., Lindo de Soriano I., Vaisberg A. In vitro studies of the role of monocytes in the immunosuppression associated with natural measles virus infections. Clin Immunol Immunopathol. 1987 Dec;45(3):375–383. doi: 10.1016/0090-1229(87)90090-0. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Huddlestone J. R., Lampert P. W., Oldstone M. B. Virus-lymphocyte interactions: infection of Tg and Tm subsets by measles virus. Clin Immunol Immunopathol. 1980 Mar;15(3):502–509. doi: 10.1016/0090-1229(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Korkiamäki P., Vainionpä R. Replication of measles virus in human lymphocytes. J Exp Med. 1985 Jun 1;161(6):1261–1271. doi: 10.1084/jem.161.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., McFarland H. F. Measles virus persistence in human lymphocytes: a role for virus-induced interferon. J Gen Virol. 1982 Dec;63(2):351–357. doi: 10.1099/0022-1317-63-2-351. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnavuori K., Hovi T. Restricted replication of herpes simplex virus in human monocyte cultures: role of interferon. Virology. 1983 Oct 15;130(1):1–9. doi: 10.1016/0042-6822(83)90112-5. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J. C., Rezee A., Galama J. M. Activation of measles virus from silently infected human lymphocytes. J Exp Med. 1978 Oct 1;148(4):940–952. doi: 10.1084/jem.148.4.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J., Galama J. M., Rezee A. Studies on the mechanism of measles virus-induced suppression of lymphocyte functions in vitro: lack of a role for interferon and monocytes. Cell Immunol. 1978 May;37(2):448–458. doi: 10.1016/0008-8749(78)90212-5. [DOI] [PubMed] [Google Scholar]
- Mayernik D. G., Haq A., Rinehart J. J. Interleukin 1 secretion by human monocytes and macrophages. J Leukoc Biol. 1984 Oct;36(4):551–557. doi: 10.1002/jlb.36.4.551. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Fujinami R. S., Lampert P. W., Oldstone M. B. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986 May 1;163(5):1331–1336. [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen K. E., Pyhälä L., Cantell K. Raising antibodies to human leukocyte interferon. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):443–450. doi: 10.1111/j.1699-0463.1975.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARR S., BERKOVICH S. EFFECTS OF MEASLES, GAMMA-GLOBULIN-MODIFIED MEASLES AND VACCINE MEASLES ON THE TUBERCULIN TEST. N Engl J Med. 1964 Feb 20;270:386–391. doi: 10.1056/NEJM196402202700802. [DOI] [PubMed] [Google Scholar]
- Salonen R., Ilonen J., Salmi A. Measles virus infection of unstimulated blood mononuclear cells in vitro: antigen expression and virus production preferentially in monocytes. Clin Exp Immunol. 1988 Feb;71(2):224–228. [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Vydelingum S., Margolese R. G. Viral inhibition of lymphocyte mitogenesis: interference with the synthesis of functionally active T cell growth factor (TCGF) activity and reversal of inhibition by the addition of same. J Immunol. 1983 May;130(5):2372–2378. [PubMed] [Google Scholar]