Abstract
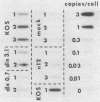
Using nonsense and deletion mutants of herpes simplex virus type 1, we investigated the roles of three immediate-early proteins (ICP4, ICP27 and ICP0) in the establishment and reactivation of ganglionic latency in a mouse ocular model. DNA hybridization, superinfection-rescue, and cocultivation techniques provided quantitative data that distinguished between the failure of a virus to establish latency in the ganglion and its failure to reactivate. Null mutants with lesions in the genes for ICP4 and ICP27 did not replicate in the eye or in ganglia and failed to establish reactivatable latent infections. Three ICP0 deletion mutants which could replicate in the eye and ganglia varied in their ability to establish and reactivate from the latent state, demonstrating that ICP0 plays a role both in the establishment and the reactivation of latency. The use of viral mutants and a variety of stage-specific assays allowed us to better define the stages in the establishment and reactivation of herpes simplex virus type 1 latency.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baichwal V. R., Sugden B. Latency comes of age for herpesviruses. Cell. 1988 Mar 25;52(6):787–789. doi: 10.1016/0092-8674(88)90419-9. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., Courtney M. A., Schaffer P. A. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J Virol. 1984 Dec;52(3):767–776. doi: 10.1128/jvi.52.3.767-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985 Aug;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987 Jun 11;15(11):4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Y. J., Rock D. L. Co-cultivation versus blot hybridization for the detection of trigeminal ganglionic latency following corneal inoculation with HSV-1 strains of varying TK expression and pathogenicity. Curr Eye Res. 1984 Sep;3(9):1097–1100. doi: 10.3109/02713688409000807. [DOI] [PubMed] [Google Scholar]
- Harbour D. A., Hill T. J., Blyth W. A. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J Gen Virol. 1983 Jul;64(Pt 7):1491–1498. doi: 10.1099/0022-1317-64-7-1491. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P. G., Lisak R. P., Raff M. C. Cell type-specific markers for human glial and neuronal cells in culture. Lab Invest. 1980 Oct;43(4):342–351. [PubMed] [Google Scholar]
- Klein R. J. Effect of immune serum on the establishment of herpes simplex virus infection in trigeminal ganglia of hairless mice. J Gen Virol. 1980 Aug;49(2):401–405. doi: 10.1099/0022-1317-49-2-401. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., DeStefano E. Latent herpes simplex virus infections in sensory ganglia of hairless mice prevented by acycloguanosine. Antimicrob Agents Chemother. 1979 May;15(5):723–729. doi: 10.1128/aac.15.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973 Sep 1;138(3):740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Tsuchiya Y., Sekizawa T., Kumagai K. Inhibition by indomethacin of in vitro reactivation of latent herpes simplex virus type 1 in murine trigeminal ganglia. J Gen Virol. 1984 Oct;65(Pt 10):1665–1674. doi: 10.1099/0022-1317-65-10-1665. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Silver S., Hubenthal-Voss J., McKnight J. L., Roizman B. Regulation of herpes simplex virus 1 genes: alpha gene sequence requirements for transient induction of indicator genes regulated by beta or late (gamma 2) promoters. Virology. 1986 Mar;149(2):152–164. doi: 10.1016/0042-6822(86)90117-0. [DOI] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J Virol. 1985 Dec;56(3):723–733. doi: 10.1128/jvi.56.3.723-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Cantin E. M., Notkins A. L. Homology between murine and human cellular DNA sequences and the terminal repetition of the S component of herpes simplex virus type 1 DNA. Cell. 1982 Nov;31(1):81–87. doi: 10.1016/0092-8674(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Puga A., Notkins A. L. Continued expression of a poly(A)+ transcript of herpes simplex virus type 1 in trigeminal ganglia of latently infected mice. J Virol. 1987 May;61(5):1700–1703. doi: 10.1128/jvi.61.5.1700-1703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985 May;5(5):957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988 Oct;62(10):3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Schaffer P. A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987 Mar;61(3):829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Homa F. L., Glorioso J. C., Levine M. Regulation of the herpes simplex virus type 1 late (gamma 2) glycoprotein C gene: sequences between base pairs -34 to +29 control transient expression and responsiveness to transactivation by the products of the immediate early (alpha) 4 and 0 genes. Nucleic Acids Res. 1987 Apr 10;15(7):3097–3111. doi: 10.1093/nar/15.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C., Tullo A. B., Hill T. J., Blyth W. A., Easty D. L. Spread of herpes simplex virus and distribution of latent infection after intraocular infection of the mouse. Arch Virol. 1985;85(3-4):175–187. doi: 10.1007/BF01314229. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Stow E. C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986 Dec;67(Pt 12):2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo A. B., Shimeld C., Blyth W. A., Hill T. J., Easty D. L. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982 Nov;63(Pt 1):95–101. doi: 10.1099/0022-1317-63-1-95. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Devi-Rao G., Feldman L. T., Dobson A. T., Zhang Y. F., Flanagan W. M., Stevens J. G. Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol. 1988 Apr;62(4):1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K., Stevens J. G., Cook M. L., Subak-Sharpe J. H. Latency competence of thirteen HSV-1 temperature-sensitive mutants. J Gen Virol. 1980 Jul;49(1):149–159. doi: 10.1099/0022-1317-49-1-149. [DOI] [PubMed] [Google Scholar]
- Whitby A. J., Blyth W. A., Hill T. J. The effect of DNA hypomethylating agents on the reactivation of herpes simplex virus from latently infected mouse ganglia in vitro. Brief report. Arch Virol. 1987;97(1-2):137–144. doi: 10.1007/BF01310742. [DOI] [PubMed] [Google Scholar]
- Wilcox C. L., Johnson E. M., Jr Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol. 1987 Jul;61(7):2311–2315. doi: 10.1128/jvi.61.7.2311-2315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]