Abstract
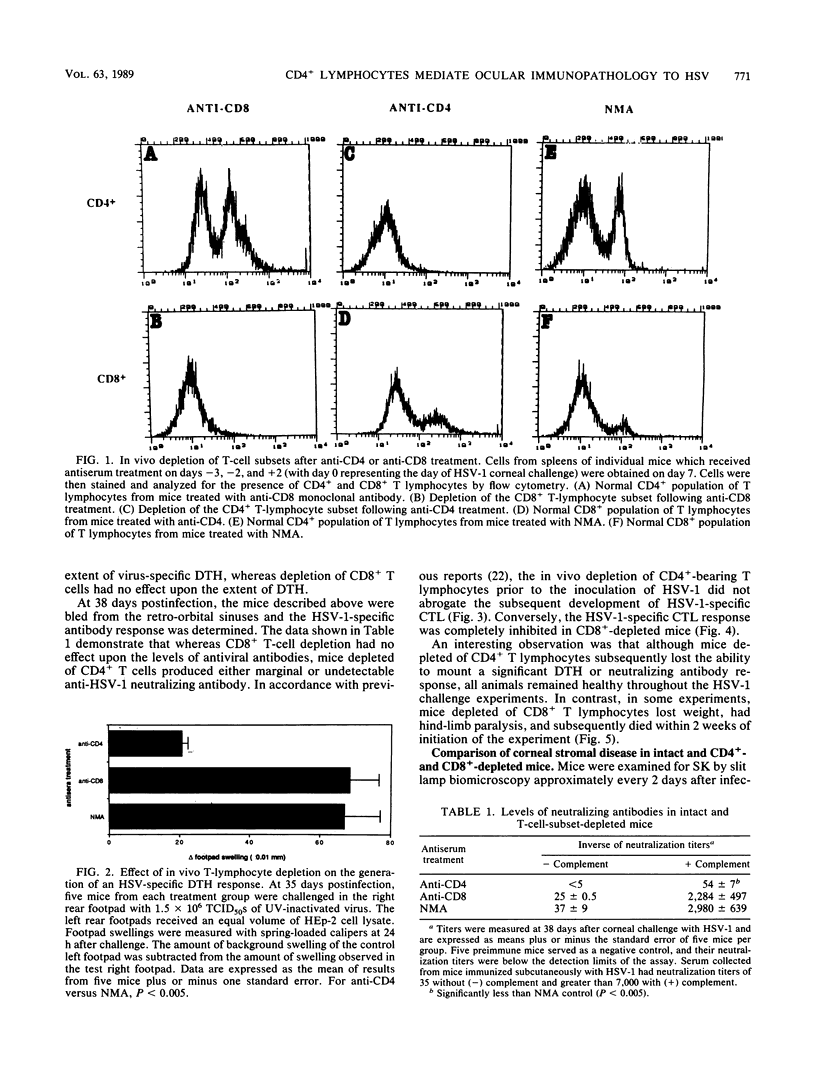
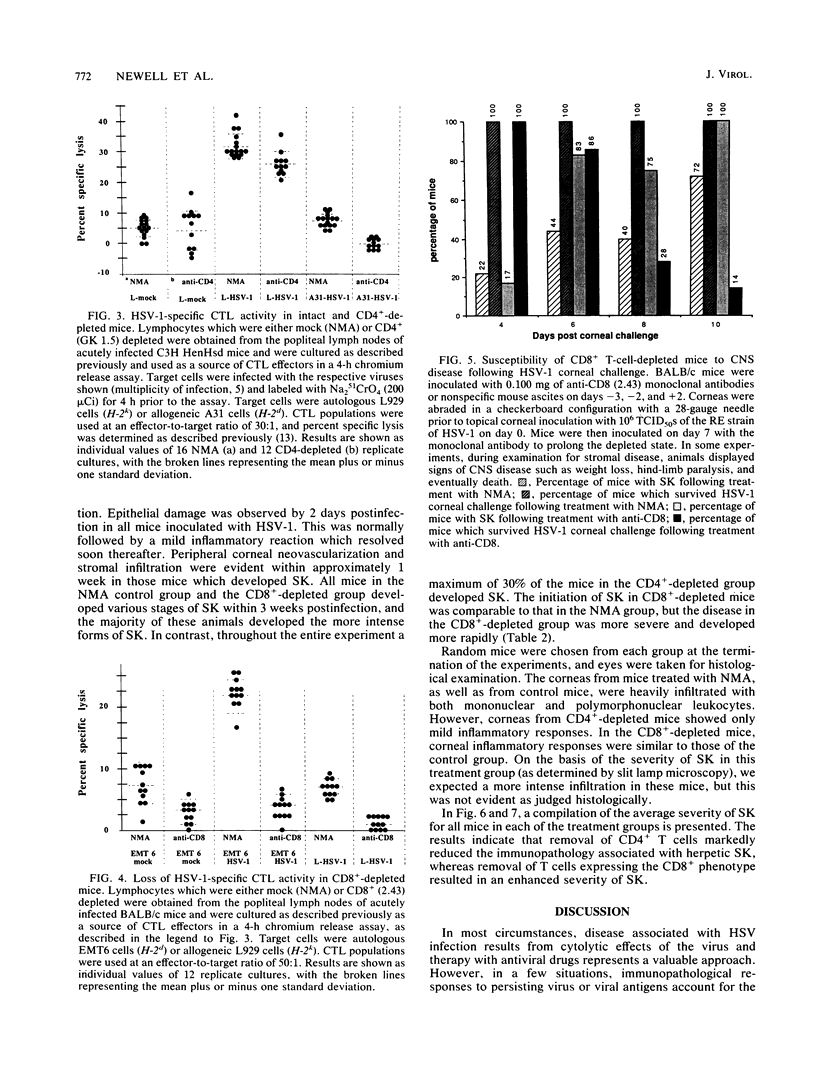
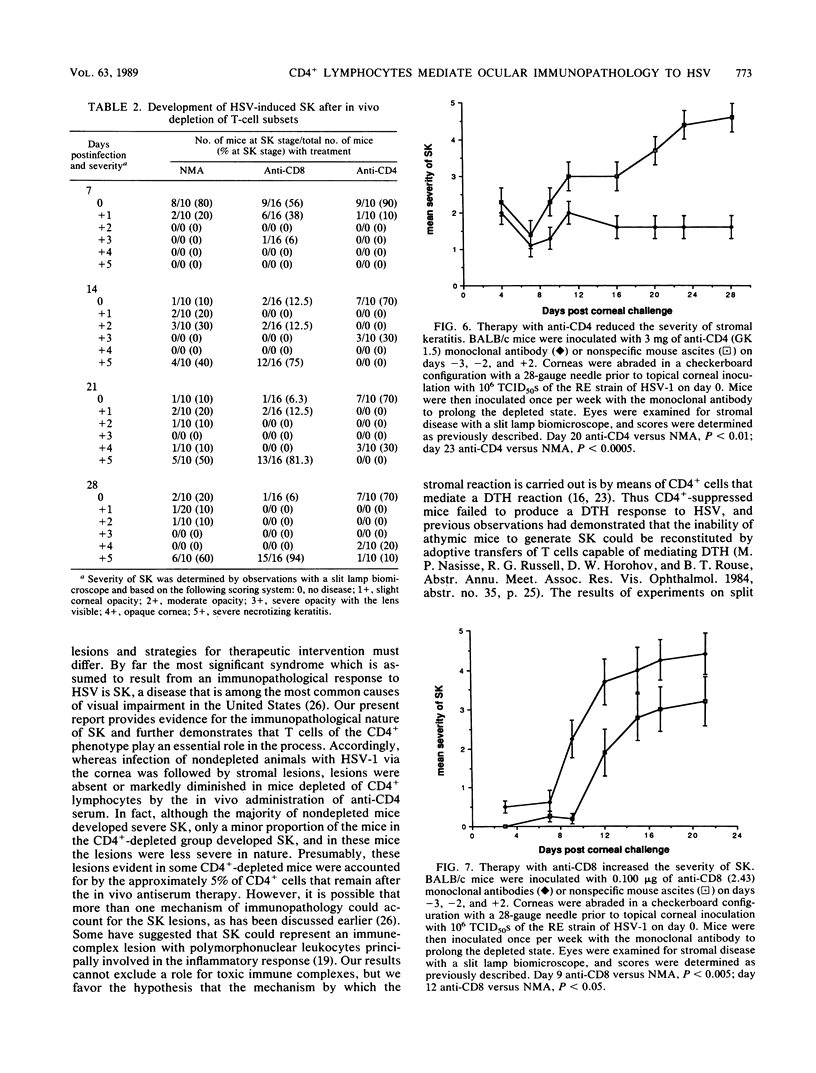
Herpetic stromal keratitis (SK), a frequent cause of visual impairment, is considered to represent an immune-mediated inflammatory response to persistent herpes simplex virus virions or subcomponents within the corneal stroma. The experimental disease in mice involves the essential participation of T lymphocytes, but the role of T-lymphocyte subsets in either mediating or controlling the disease is uncertain. In this report, rat monoclonal antibodies were used to selectively deplete mice in vivo of CD4+ (helper-inducer) and CD8+ (cytotoxic-suppressor) T-cell populations and the effect on herpetic SK was evaluated. As measured by flow cytometry, mice treated with anti-CD4 monoclonal antibody (GK 1.5) were greater than 95% depleted of CD4+ T lymphocytes and mice treated with anti-CD8 monoclonal antibody (2.43) were 90% depleted of CD8+ T lymphocytes. Depleted and nonspecific mouse ascites-treated control mice were infected topically on the corneas with herpes simplex virus type 1, and the induction of various immune parameters during the acute infection was evaluated. CD4+-depleted mice failed to produce either a significant antiviral antibody or delayed-type hypersensitivity response but were capable of producing normal cytotoxic T-lymphocyte responses. In contrast, CD8+-depleted mice produced antiviral antibody and delayed-type hypersensitivity responses comparable with those in control animals, but cytotoxic T-lymphocyte responses were markedly reduced. Clinical observations of the corneas revealed that SK in CD4+-depleted mice was significantly reduced, whereas in CD8+-depleted mice SK developed more rapidly, was more severe, and involved a greater percentage of mice. These observations implicate the CD4+ T-lymphocyte subset as the principal mediators of SK and CD8+ T lymphocytes as possible regulators that control the severity of SK.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Blyth W. A. Lipopolysaccharide-induced suppressor cells for delayed-type hypersensitivity to herpes simplex virus: nature of suppressor cell and effect on pathogenesis of herpes simplex. Immunology. 1984 Nov;53(3):473–480. [PMC free article] [PubMed] [Google Scholar]
- Aronson S. B., Moore T. E., Jr Corticosteroid therapy in central stromal keratitis. Am J Ophthalmol. 1969 Jun;67(6):873–896. doi: 10.1016/0002-9394(69)90082-8. [DOI] [PubMed] [Google Scholar]
- Chandler J. W. Immunologic mechanisms involved in viral ocular diseases. Ophthalmology. 1980 Dec;87(12):1239–1243. doi: 10.1016/s0161-6420(80)35102-6. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Easty D. L., Maini R. N., Jones B. R. Cellular immunity in herpes simplex keratitis. Trans Ophthalmol Soc U K. 1973;93(0):171–180. [PubMed] [Google Scholar]
- KAUFMAN H., MARTOLA E. L., DOHLMAN C. Use of 5-iodo-2'-deoxyuridine (IDU) in treatment of herpes simplex keratitis. Arch Ophthalmol. 1962 Aug;68:235–239. doi: 10.1001/archopht.1962.00960030239015. [DOI] [PubMed] [Google Scholar]
- Ksander B. R., Hendricks R. L. Cell-mediated immune tolerance to HSV-1 antigens associated with reduced susceptibility to HSV-1 corneal lesions. Invest Ophthalmol Vis Sci. 1987 Dec;28(12):1986–1993. [PubMed] [Google Scholar]
- Larsen H. S., Feng M. F., Horohov D. W., Moore R. N., Rouse B. T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984 Apr;50(1):56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathey J. L., Martin S., Rouse B. T. Suppression of delayed type hypersensitivity to herpes simplex virus type 1 following immunization with anti-idiotypic antibody: an example of split tolerance. J Gen Virol. 1987 Apr;68(Pt 4):1093–1102. doi: 10.1099/0022-1317-68-4-1093. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Different functions of subsets of effector T cells in murine influenza virus infection. Cell Immunol. 1982 Mar 1;67(2):312–324. doi: 10.1016/0008-8749(82)90223-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Inhibition of pathogenic effect of effector T cells by specific suppressor T cells during influenza virus infection in mice. Nature. 1983 Aug 11;304(5926):541–543. doi: 10.1038/304541a0. [DOI] [PubMed] [Google Scholar]
- Martin S., Rouse B. T. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J Immunol. 1987 May 15;138(10):3431–3437. [PubMed] [Google Scholar]
- Metcalf J. F., Hamilton D. S., Reichert R. W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979 Dec;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Kaufman H. E. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976 Dec;82(6):827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Meyers-Elliott R. H., Chitjian P. A. Immunopathogenesis of corneal inflammation in herpes simplex virus stromal keratitis: role of the polymorphonuclear leukocyte. Invest Ophthalmol Vis Sci. 1981 Jun;20(6):784–798. [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G., Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: simultaneous induction of protective immunity and tolerance to delayed-type hypersensitivity. Immunology. 1981 May;43(1):153–159. [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Pfizenmaier K., Starzinski-Powitz A., Röllinghoff M., Falks D., Wagner H. T-cell-mediated cytotoxicity against herpes simplex virus-infected target cells. Nature. 1977 Feb 17;265(5595):630–632. doi: 10.1038/265630a0. [DOI] [PubMed] [Google Scholar]
- Russell R. G., Nasisse M. P., Larsen H. S., Rouse B. T. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):938–944. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Tolerance and suppression of immunity to herpes simplex virus: different presentations of antigens induce different types of suppressor cells. Infect Immun. 1983 May;40(2):514–522. doi: 10.1128/iai.40.2.514-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swyers J. S., Lausch R. N., Kaufman H. E. Corneal hypersensitivity to herpes simplex. Br J Ophthalmol. 1967 Dec;51(12):843–846. doi: 10.1136/bjo.51.12.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS L. E., NESBURN A. B., KAUFMAN H. E. EXPERIMENTAL INDUCTION OF DISCIFORM KERATITIS. Arch Ophthalmol. 1965 Jan;73:112–114. doi: 10.1001/archopht.1965.00970030114023. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]


