Abstract
Cancer relapse after surgery is a common occurrence, most frequently resulting from the outgrowth of minimal residual disease in the form of metastases. We examined the effectiveness of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade as an adjunctive immunotherapy to reduce metastatic relapse after primary prostate tumor resection. For these studies, we developed a murine model in which overt metastatic outgrowth of TRAMP-C2 (C2) prostate cancer ensues after complete primary tumor resection. Metastatic relapse in this model occurs reliably and principally within the draining lymph nodes in close proximity to the primary tumor, arising from established metastases present at the time of surgery. Using this model, we demonstrate that adjunctive CTLA-4 blockade administered immediately after primary tumor resection reduces metastatic relapse from 97.4 to 44%. Consistent with this, lymph nodes obtained 2 weeks after treatment reveal marked destruction or complete elimination of C2 metastases in 60% of mice receiving adjunctive anti-CTLA-4 whereas 100% of control antibody-treated mice demonstrate progressive C2 lymph node replacement. Our study demonstrates the potential of adjunctive CTLA-4 blockade immunotherapy to reduce cancer relapse emanating from minimal residual metastatic disease and may have broader implications for improving the capability of immunotherapy by combining such forms of therapy with other cytoreductive measures including surgery.
Cancer outgrowth from residual disease, most commonly in the form of metastases, represents the principle mechanism of relapse after solid-tumor surgery. Moreover, for patients who present with locally advanced tumors or small-volume metastases, surgery is often eliminated as a potential treatment option because of the high risk for outgrowth of residual disease after primary tumor resection. Hence, intense attention has been given to adjunctive therapies that demonstrate the potential to eradicate “minimal residual disease” to prevent cancer relapse after surgery. One general form of therapy that might be a useful adjunctive treatment is immunotherapy.
In vivo blockade of the T cell inhibitory receptor, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), using anti-CTLA-4 antibody (CTLA-4 blockade) may represent a particularly practical immunotherapeutic adjunct to cancer surgery. CTLA-4 blockade has been demonstrated to potentiate antitumor immune responses against a number of experimental cancers, including murine colorectal carcinoma (51Blim10), fibrosarcoma (Sa1N) (1), and the transgenic adenocarcinoma of mouse prostate (TRAMP)-derived prostate cancer cell line, TRAMP-C1 (2, 3). More recently, it has been shown that antitumoral responses raised by CTLA-4 blockade can be enhanced further by combining the administration of anti-CTLA-4 antibodies with tumor cell vaccines that are intended to boost host tumor-antigen presentation (4, 5).
The mechanism whereby CTLA-4 blockade boosts antitumoral immunity relates to the inhibitory role of CTLA-4 in the T cell costimulatory pathway. It is now clear that two signals are essential for the full induction of antigen-specific T cell activation (6). The first “antigen-specific” signal arises from interactions between MHC/antigen complexes with the T cell receptor. The second antigen-nonspecific “costimulatory” signal arises from interactions between the costimulatory family of B7 (CD80 or CD86) ligands and T cell CD28. CTLA-4, which competes with CD28 to bind B7, has been shown act as a counter-regulatory receptor that attenuates T cell responses by preventing full T cell activation or terminating T cell responses (reviewed in refs. 7 and 8). Consistent with this, it has been shown that CTLA-4 blockade can potentiate T cell responses by prolonging T cell activity and/or facilitating antigen-specific T cell costimulatory activation (7, 8).
In the present study, we test the hypothesis that CTLA-4 blockade can be used as an adjunctive form of immunotherapy to eliminate residual prostate cancer metastases after primary tumor removal. For these studies, an immunocompetent model that nominally recapitulates clinical metastatic cancer relapse after complete primary tumor resection was developed. The establishment of this model is significant because, in general, the development of adjunctive cancer therapies has been markedly hindered by the absence of animal models that mimic metastatic disease relapse after complete primary tumor removal. We demonstrate that the TRAMP-derived murine prostate cancer cell line, TRAMP-C2 (C2), is not only tumorigenic as we previously reported (3), but also metastasizes to regional lymph nodes, submandibular salivary gland and lungs after a chronic interval of primary tumor growth. C2 primary tumors can be completely resected with a very low frequency of local recurrence. After primary tumor removal, nearly all mice experience metastatic relapse arising from established micrometastases that are present at the time of primary tumor resection. The primary site of metastatic C2 relapse in this model is the regional draining lymph nodes in close proximity to the primary tumor. Using this model, we demonstrate that CTLA-4 blockade, when administered as an adjunctive form of immunotherapy, can reduce the incidence of metastatic relapse by causing the elimination of established micrometastases already present at the time of surgery. Finally, we provide some histologic evidence that the elimination of these micrometastases might be mediated by a cellular immune response raised by adjunctive CTLA-4 blockade.
Materials and Methods
Growth and Maintenance of Cell Lines.
The C2 cell line used in these studies is an early passage line derived from the TRAMP mouse that spontaneously develops autochthonous tumors attributable to prostate-restricted SV(40) T antigen (Tag) expression (9). Consistent with its prostate epithelial origin, C2 expresses androgen receptor, E-cadherin, and cytokeratin. Roughly one-third of in vivo C2 tumors also express probasin, a secretory protein that is specifically elaborated by the luminal epithelial cells of the rodent prostate (3). C2 cells used in these studies were cultured and maintained as described (2, 3). Passages 9–20 were used for the present studies. Before injection of C2 into syngeneic male C57BL/6 mice, cell suspensions were washed three times in serum-free DMEM. Mice were injected with 2.5–5 × 106 cells in 0.1 ml of serum-free DMEM. Injections were delivered subcutaneously between the scapulae by using a 19-gauge needle.
Anti-CTLA-4 Production, Purification, and Titering.
Anti-CTLA-4 antibody used in these studies was protein G-purified from supernatants from the 9H10 hybridoma line raised in a Cell Pharm System 2000 Bioreactor (UniSyn, Boston). Antibody concentrations were quantified by ultraviolet spectrophotometry as previously described (1).
Animal Surgery and Tumor Kinetic Studies.
Animal experiments were conducted in the Laboratory of Kidney and Electrolyte Metabolism, National Heart Lung and Blood Institute, in accordance with National Institutes of Health Animal Care and Use Guidelines. Six- to eight-week old male C57BL/6 mice were obtained from The Jackson Laboratory. When tumor base area achieved ≥250 mm2, ≈6 weeks after C2 cell injection, mice were anesthetized and tumors were resected. In Experiment 1, C2 tumors were removed by sharply incising the skin immediately adjacent to the tumor base and then shelling the tumor out from its investing tissues. To further diminish local tumor recurrence, in subsequent experiments (2 and 3) the surgical procedure was modified by widening the resection margin around the tumor to 0.5 cm. Additionally, the anterior limit of tumor resection was extended to the fascia of the back musculature, and the lateral margins were extended to, but did not include, the axillary lymph nodes. All investing tissues were removed en bloc with the tumor left undisturbed within these tissues. After tumor resection, the skin was closed with stainless steel clips. Mice were then randomized into treatment cohorts. During randomization, special care was taken to ensure that cohorts were represented by a similar number of equal-sized tumors that had been resected. Treatment cohorts received intraperitoneal antibody injections (100 μg) of either anti-CTLA-4 (blockade) or isotype-matched IgG, on days 4, 7, and 10 after surgery. Tumor recurrences at the primary site and within regional and juxta regional lymph nodes were quantified by using vernier calipers by obtaining bisecting measurements of recurrent lesions (recorded as square millimeters). Incidences of tumor relapse for control and anti-CTLA-4 treated cohorts were compared for statistically significant differences (P < 0.05) by Student's t test analysis (graphpad instat 3.00, GraphPad, San Diego).
Histopathologic Analysis of Tissues.
Immediately after the killing of mice by cervical dislocation, recurrent primary tumors, axillary and anterior cervical lymph nodes, salivary glands, and lungs were harvested for histologic examination. These tissues were fixed overnight in zinc-buffered formalin and were paraffin-embedded. Additionally, in some studies, tissues were snap-frozen in OCT compound to permit immunohistologic analysis. Paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E). To aid in the detection of micrometastases, frozen lymph nodes were immunohistologically stained for cytokeratin by using pan cytokeratin antibody (clone Z0622, Dako) at a 1:1,000 dilution, as described (3).
Results
In Vivo CTLA-4 Blockade Slows C2 Growth or Causes Its Rejection In C57BL/6 Male Mice.
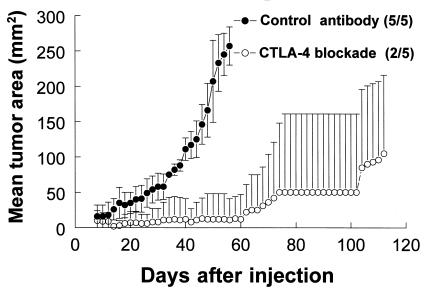
We have previously shown that in vivo CTLA-4 blockade can slow the growth, or elicit the rejection, of TRAMP-C1 subcutaneous tumors by the syngeneic nontransgenic C57BL/6 host (2). In the present study, we show that in vivo CTLA-4 blockade can elicit a similar response against C2 (Fig. 1). Like TRAMP-C1, C2 is an early passage prostate cancer cell line that was originally derived from the TRAMP mouse. In three separate experiments, a total of 73% (11/15) of mice injected with 2.5 × 106 C2 cells were rendered tumor-free for ≥100 days after treatment with CTLA-4 blockade (anti-CTLA-4, 100 μg, i.p., given on days 7, 10, and 13 after tumor cell challenge) whereas the remaining 27% (4/15) of these mice experienced progressive C2 tumor outgrowth. In contrast, 100% (15/15) of mice injected with control antibody (irrelevant hamster antibody) experienced progressive C2 tumor outgrowth (P < 0.05, comparison of tumor incidence for anti-CTLA-4 versus control-treated mice). Thus, in vivo CTLA-4 blockade can raise an antitumoral response sufficient to eradicate C2 after tumor cell challenge.
Figure 1.
In vivo CTLA-4 blockade slows or prevents the outgrowth of C2 tumors. C57BL/6 mice were injected with 2.5 × 106 C2 cells. Data are mean tumor areas from a single representative of three separate experiments in which groups of five mice received 100-μg intraperitoneal injections of either a control antibody or anti-CTLA-4 (CTLA-4 blockade) on days 7, 10, and 13 after tumor cell injection. The fraction of animals in each group that formed tumors is provided in parentheses next to corresponding marker designations. Note that the lower error bars for CTLA-4 blockade-treated mice have been omitted for clarity.
C2 Primary Tumor Outgrowth Is Accompanied by the Establishment of Regional Lymph Nodes Metastases.
In light of the capability of CTLA-4 blockade to elicit a response against prostate tumors in the subcutaneous murine model, it was of particular interest to test whether CTLA-4 blockade might be an effective adjunctive treatment to eradicate metastases left in situ after primary tumor removal. To test this, and given that a primary route of prostate cancer dissemination in humans is via lymphatic spread, it was first necessary to develop a model that mimics the proclivity of prostate cancer to metastasize to regional lymph nodes.
Shortly after reporting the tumorigenicity of the C2 (3), we observed that C2 lymph node metastases could frequently be found in proximity to the primary tumor, especially when the primary tumor was very large. Hence, to systematically assess the incidence of C2 metastases to draining lymph nodes, the following was performed. Thirty C57BL/6 mice received subcutaneous dorsal neck injections with 5 × 106 C2 cells. When C2 tumors achieved ≥250 mm2, mice were killed, and regional axillary and anterior cervical lymph nodes were harvested. Lymph nodes from these mice were first screened for the presence of C2 metastases by microscopic examination of five serial H&E-stained sections obtained from the center of nodal specimens. Screening by this method revealed that 24/30 mice (80%) harbored C2 metastases (Fig. 2A). An additional, five mice harbored C2 lymph node metastases that were only demonstrable by cytokeratin staining of additional tissue sections (data not shown). C2 lymph node metastases ranged from <0.1 to 2.8 mm in greatest dimension with a mean size 0.88 ± 0.66 mm (greatest mean diameter ± SD). Nineteen of twenty-nine (66%) mice harbored lymph nodes containing metastases <1 mm in size. Hence, overall, 97% of these C2 tumor-bearing mice (29/30) harbored established regional lymph node metastases. These data illustrate the metastatic capability of C2 and that C2 primary tumor outgrowth results in the establishment of regional lymphatic metastases in a high percentage of mice.
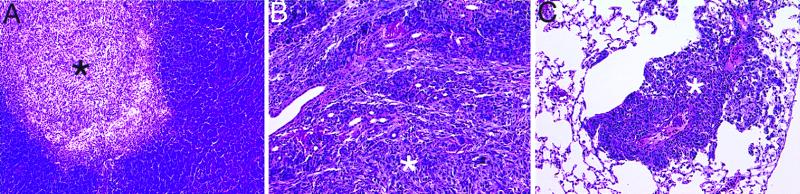
Figure 2.
Histology of C2 metastases stained with H&E. (A) Typical C2 metastasis (*) within a lymph node harvested from a mouse bearing a 250-mm2 C2 primary tumor. C2 infiltration of the serous submandibular salivary gland (B) and metastasis to the lung (C) after overt C2 metastatic lymph node relapse following primary tumor resection and administration of control antibody. All sections are shown at ×20.
Adjunctive CTLA-4 Blockade Immunotherapy Reduces Metastatic Failure After Primary Tumor Resection.
To test the hypothesis that adjunctive CTLA-4 blockade can be used to prevent or reduce treatment failure caused by outgrowth of residual established metastases after surgery, the following studies were performed. In three separate experiments, groups of C57BL/6 mice underwent resection of their dorsal-neck primary C2 tumors when tumor size achieved ≥250 mm2 (Experiment 1, 9 mice/cohort; Experiments 2 and 3, 15 mice/cohort). On days 4, 7, and 10 after tumor resection, mice were randomized to receive intraperitoneal injections of either anti-CTLA-4, or control antibody. After surgery, mice were followed for overt (visible on inspection) local and/or metastatic C2 outgrowth. Follow-up of mice was typically begun ≈21 days after surgery because, before this time, assessment of potential relapse sites was hampered by distortions caused by the surgical wound. Mice were killed when the aggregate size of recurrent tumor (primary tumor plus metastatic tumor size) achieved or exceeded 250 mm2.
The incidences and distribution of C2 recurrences in individual experiments for both control and CTLA-4 blockade-treated mice are presented in Table 1. For the three experiments overall, 97.4% (38/39) of control-treated mice suffered overt metastatic C2 progression, typically within 40 days after primary tumor removal. Metastatic C2 outgrowth was observed at axillary and/or anterior cervical lymph node sites in all control-treated mice that failed whereas 23% (9/39) of mice had additional local tumor recurrences. However, most of the mice that experienced local tumor recurrence (6/9) originated from Experiment 1, in which a relatively conservative approach to primary tumor resection was used. Histopathologic analysis further revealed that 53% (21/39) of control-treated mice harbored additional micrometastases within the serous submandibular glands (Fig. 2B) and/or lungs (Fig. 2C).
Table 1.
Incidences and sites of C2 metastasis/recurrence after treatment
| Adjuvant treatment | Regional lymph nodes | Resection site | Lung and/or salivary gland |
|---|---|---|---|
| Control IgG | |||
| Experiment 1 | 9/9 (100) | 6/9 (67) | 4/9 (44) |
| Experiment 2 | 14/15 (93) | 3/15 (20) | 8/15 (53) |
| Experiment 3 | 15/15 (100) | 0/15 (0) | 9/15 (60) |
| CTLA-4 blockade | |||
| Experiment 1 | 4/9 (44) | 2/9 (22) | 2/9 (22) |
| Experiment 2 | 5/14 (36) | 1/14 (7) | 4/14 (29) |
| Experiment 3 | 7/15 (47) | 0/15 (0) | 3/15 (20) |
After resection of C2 primary tumors and adjunctive treatment with either control IgG or anti-CTLA-4 (CTLA-4 blockade), mice were monitored for ≥150 days for overt tumor outgrowth at regional lymph node sites (axillary or anterior cervical nodes) or the tumor resection site. When aggregate tumor dimensions achieved 250 mm2, mice were killed, and regional lymph nodes, lungs, and salivary glands were harvested. Lungs and salivary glands were fixed and stained by routine H&E to assess the presence of distant microscopic metastases to these organs. Data are the number of mice that had C2 metastases (reported according to site of failure) over the total number of mice in the treatment cohort. Percentages of mice with metastases (reported according to site of failure) are given in parentheses.
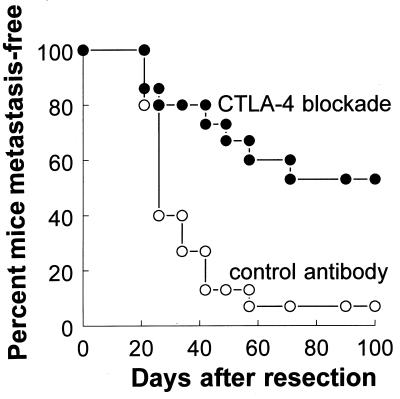
In contrast, mice treated with adjunctive anti-CTLA-4 experienced far fewer treatment failures (Table 1; Fig. 3). Overall, only 44% (17/38) of anti-CTLA-4-treated mice experienced C2 progression after primary tumor resection (Table 1)—significantly less than the overall relapse rate of 97.4% observed for mice receiving control antibody (P < 0.05). As was the case for control-treated mice, virtually all CTLA-4 blockade-treated mice failing treatment demonstrated C2 outgrowth at axillary and/or anterior cervical lymph node sites, with one mouse (Experiment 2) experiencing a local tumor recurrence only. Local tumor recurrences and distant (salivary and lung) micrometastases were observed in only 8% (3/38) and 24% (9/38) of these mice, respectively. Thus, although CTLA-4 blockade did lower treatment failures overall, it did not markedly alter the distribution of sites of failure. For the remaining 56% (21/38) mice that responded to adjunctive CTLA-4 blockade, overt C2 outgrowth did not occur even after >150 days of follow-up. Moreover, detailed histologic evaluation of lymph nodes, salivary glands, and the lungs of these mice failed to reveal any evidence of residual C2 metastases. Finally, although a comparison of H&E sections from anti-CTLA-4-treated and control mice (from Experiments 1 and 2) failed to reveal obvious prostate lymphocytic infiltration after adjunctive CTLA-4 blockade, more rigorous immunohistologic studies will be needed to assess the potential of CTLA-4 blockade to induce prostate autoimmunity in our model.
Figure 3.
Effect of adjunctive CTLA-4 blockade on tumor-free survival of mice after surgical resection of primary C2 tumors. Primary C2 tumors were resected from the dorsal neck of male C57BL/6 mice on day 0 as described in Materials and Methods. Subsequently, cohorts of mice were randomized to receive intraperitoneal injections of 100 μg of either anti-CTLA-4 (CTLA-4 blockade) or control antibody on days 4, 7, and 10 after primary tumor resection (15 mice per treatment group). Beginning on day 21 after surgery, mice were evaluated for the presence of overt (visible) tumors arising from axillary and/or anterior cervical lymph nodes or the primary resection site. Mice not experiencing C2 relapse were followed for a minimum of 150 days after treatment. Data are the percent of mice remaining tumor-free (vertical axis) as a function of the number of days after primary tumor resection (horizontal axis). The experiment shown is a single representative (Experiment 2) of three separate experiments. In this experiment, one mouse in the CTLA-4 blockade-treated cohort died immediately after surgery.
Adjunctive CTLA-4 Blockade Reduces Surgical Failure by Causing the Elimination of Established C2 Metastases.
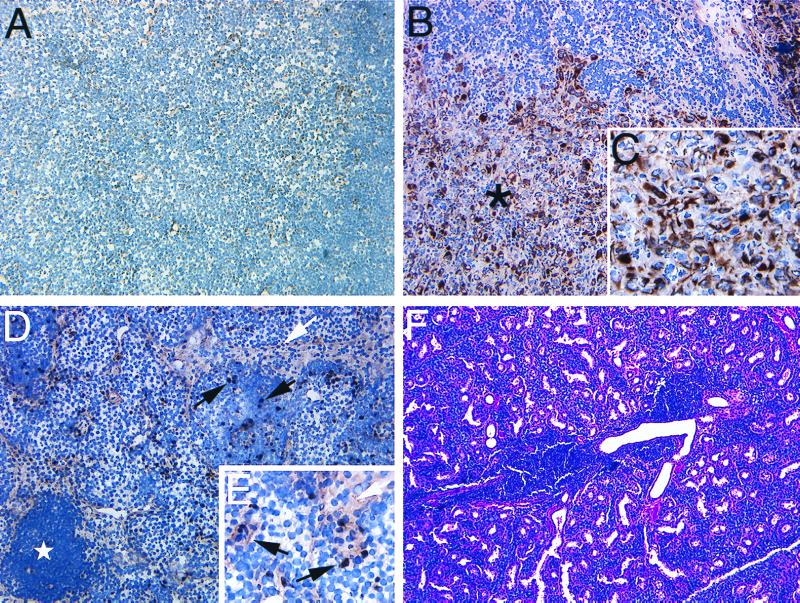
To further test whether adjunctive CTLA-4 blockade decreases metastatic failure by causing the elimination of residual C2 metastases after surgery, the following experiment was performed. Fifteen-mouse cohorts underwent primary C2 tumor resection and treatment with either CTLA-4-blockade or control antibody. A cohort of non-tumor-bearing mice was sham-operated and included as an additional control group for this study. At 2 weeks after the last day of antibody treatment, axillary and anterior cervical lymph nodes from mice in these cohorts were stained for cytokeratin-positive C2 metastases. Lymph nodes recovered from sham-operated control mice demonstrated extremely weak and nonspecific cytokeratin staining of stromal elements (Fig. 4A) whereas 100% (15/15) of control tumor-resected mice harbored obvious, intensely cytokeratin-positive, C2 metastases that frequently resulted in total replacement of normal lymph node architecture (Fig. 4 B and C).
Figure 4.
Histology of tissues recovered from mice after sham-surgery, or primary tumor resection followed by adjunctive antibody treatment. (A) Two weeks after sham-surgery and antibody treatment of tumor-naive mice, axillary and anterior cervical lymph nodes in proximity to the surgical site were stained for cytokeratin. Weakly cytokeratin-positive stromal epithelial cells can be observed diffusely distributed throughout the lymph nodes of these mice. (B) Intensely cytokeratin-positive C2 metastases (∗) are observed replacing the normal architecture of lymph nodes taken from mice 2 weeks after C2 primary tumor resection and adjunctive treatment with control-antibody. (C) C2 metastasis, ×40. (D) Regional lymph node recovered from mouse 2 weeks after C2 primary tumor resection and CTLA-4 blockade contains scattered cells of intense or intermediate cytokeratin staining. Marked activation of lymph nodes from these mice is evidenced by the presence of follicle (⋆) formation. Intensely cytokeratin-positive cells (black arrows) appear to represent pyknotic C2 cells whereas the large vacuolated complexes of less-intense cytokeratin staining (white arrows) may represent dying C2 cells, phagocytic cells containing tumor debris, or reactive hyperplasia of stromal elements. (E) Lymph node from mouse treated with primary C2 tumor resection and adjunctive CTLA-4 blockade, ×40. (F) Serous submandibular glands of some mice (5/15) treated with adjunctive CTLA-4 blockade contain single or multiple perivascular infiltrates composed of homogenous mononuclear cells. In contrast, mice treated with adjunctive control antibody demonstrate normal glandular morphology without attendant infiltrate formation. Unless otherwise specified, all sections shown at ×20.
In contrast, 60% (9/15) of CTLA-4 blockade-treated mice harbored lymph nodes containing only scattered pyknotic cytokeratin-positive cells (5 mice; Fig. 4 D and E) or no cytokeratin-positive cells (4 mice; data not shown). In some of these lymph nodes, large and often vacuolated cell complexes of less-intense cytokeratin staining could also be found (Fig. 4 D and E). Although it appears that these complexes represent dying islands of C2 tumor cells, it is also possible that these complexes represent phagocytic cells containing tumor cell debris or a hyperplastic response by stromal lymph node elements. Dense aggregates of lymphocytes were also commonly observed in association with these cell complexes (Fig. 4D) whereas similar aggregates were not present in any of the lymph nodes recovered from sham- or control-treated mice. For the remaining 40% (6/15) of anti-CTLA-4 treated mice, C2 lymph node metastases were readily apparent, and lymph nodes from these mice did not differ significantly from nodes recovered from control-treated tumor-resected mice.
Finally, in 5/15 (33%) mice treated with adjunctive CTLA-4 blockade, single or multiple perivascular mononuclear cell infiltrates could be identified within the serous submandibular salivary glands of these animals (Fig. 4F). Similar infiltrates were absent in glands of control mice. Given that the serous submandibular gland is a secondary site of metastasis in about one-half of mice that experience C2 metastatic outgrowth after tumor resection, and that the perivascular infiltrate that we observed appears to be lymphocytic, it is possible that this process might represent a cellular effector immune response raised against C2 metastases by CTLA-4 blockade. Hence, adjunctive CTLA-4 blockade appears to reduce surgical treatment failure by causing the elimination of established C2 metastases left in situ after primary tumor resection.
Discussion
For some cancers, including testicular cancer as a striking example, the addition of an appropriate adjunctive treatment to surgery has led to marked improvements in treatment outcomes. For prostate cancer, however, conventional adjuncts, including chemo- and radiation therapy, confer little or no advantage over surgery alone. This is of particular concern, given that roughly one-third of prostate cancer patients experience postsurgical treatment failure because of progression of residual metastases. Moreover, immediately after surgery, many of these patients will have serum prostate-specific antigen values ranging from 0.0 to 0.5 ng/ml, which correspond to a volume of 0.5 ml or less of residual cancer (10). Based on the ineffectiveness of conventional adjunctive therapies, combined with the extremely small volume of residual disease that causes treatment failure, it has been suggested that immunotherapy might prove a useful adjunct to prostate cancer surgery (11). It has further been suggested by some investigators that immunotherapy might be most effective when used as an adjunct after surgery or other cytoreductive measures (12).
The testing and development of various immunotherapies as adjuncts to surgery, however, has been generally hindered by the lack of syngeneic immunocompetent cancer models that recapitulate postsurgical metastatic cancer progression as is observed in the clinical setting. It is for this reason that we established the murine C2 tumor resection-metastasis model. This model readily lends itself to the testing of adjunctive regimens for their ability to eliminate minimal residual metastatic disease after surgery. Furthermore, this model mimics the clinical paradigm in which local recurrences account for ≈10% and metastatic cancer progression comprises the remaining 90% of treatment failures after prostate cancer surgery. The resectability of primary tumors afforded by this model permits a true assessment of the effectiveness of adjunctive therapy against well established prostate cancer metastases that develop consequent to chronic primary tumor outgrowth. Metastatic failure in our model can be readily quantified by measuring tumor-bearing axillary and cervical lymph nodes and can be followed longitudinally, obviating the need to kill cohorts of animals at various intervals to assess metastatic tumor burden.
Using this model, we demonstrate the capability of adjuvant CTLA-4 blockade to reduce surgical failure caused by metastatic relapse after primary tumor resection. The impetus for selecting CTLA-4 blockade as an adjunct to surgery is provided by its unique practicality for clinical application, and multiple prior demonstrations that CTLA-4 blockade can elicit antitumoral immune responses against a number of subcutaneous murine tumors (1, 4, 5), including murine TRAMP-C1 prostate tumors (2). We demonstrate that adjunctive CTLA-4 blockade can improve overall cancer-free survival after surgery by ≈50%. We further show that adjunctive CTLA-4 blockade appears to reduce surgical treatment failure by raising a response that culminates in the destruction of established C2 lymph node metastases. Associated with this, roughly one-third of anti-CTLA-4-treated mice develop perivascular, mononuclear cell infiltrates within their salivary glands, which serves as a secondary site for C2 metastasis in our model. Although these responses against C2 metastases can be observed within a few weeks after adjunctive anti-CTLA-4 treatment, and CTLA-4 blockade reportedly acts by boosting host T cell-mediated antitumoral immunity, further studies will be required to elucidate the precise host response that is raised against C2 metastases in our model.
A number of mechanisms may contribute the effect of CTLA-4 blockade to reduce surgical failures in our model. Potentiation of antitumoral T cell activity by CTLA-4 blockade, combined with a marked reduction in cancer burden by surgery, might simply increase the number of activated T cell effectors relative to tumor cell targets, thus favoring the elimination of metastases. Alternatively, reductions in tumor burden by surgery may facilitate a response to CTLA-4 blockade by partially or completely alleviating impairments in T cell function that can be induced by the tumor (13–21). Finally, it is worthy to note that C2 metastatic outgrowth in our model becomes particularly evident after primary tumor resection. For certain tumors, it has been suggested that the primary tumor may suppress the growth of metastases by preventing tumor vasculature formation at metastatic sites. Given that the response that is raised by CTLA-4 blockade in our model is being directed against metastases typically <1 mm in greatest dimension—at a time just before the “angiogenic switch” occurs (22, 23)—it is interesting to consider whether CTLA-4 blockade might reduce metastatic relapse by inhibiting angiogenesis. Such a mechanism has been shown to contribute to the antitumoral effects of IL-12, which enhances the production of IFN-γ, which in turn induces the production of the chemokine IP-10, a potent inhibitor of angiogenesis (24). Like IL-12, CTLA-4 blockade has also been shown to enhance INF-γ production in some models (25). Further studies will be necessary to determine whether such mechanisms mediate or contribute to the metastatic tumor destruction observed in our model.
In summary, we introduce a tumor resection-metastases model that readily lends itself to the testing of adjunctive cancer treatments, including immunotherapy, and to studies examining biological relationships that could potentially exist between the primary tumor, the host immune system, and metastases. Using this model, we demonstrate the potential of adjunctive CTLA-4 blockade immunotherapy to reduce postsurgical relapse caused by tumor outgrowth from residual metastases. Further development of this, and other adjunctive therapies, may have far-reaching implications for improving treatment outcome after prostate cancer surgery, and cancer surgery in general. Ultimately, the development of highly effective adjuvant treatments may provide a means to achieve curative responses in patients who cannot be cured by a single treatment approach alone.
Acknowledgments
This work was supported in part by National Institutes of Health/National Cancer Institute Grants CA82185 (E.D.K), S.P.O.R.E. CA58204, CA64851, and CA73747 (N.M.G), a CaPCURE Award (J.P.A.), and a generous donation to the National Heart, Lung, and Blood Institute Gift Fund by Dr. Stephen M. Quinlan. J.P.A. is an Investigator of the Howard Hughes Medical Institute. A.A.H. is a prior fellow of the Department of Defense Breast Cancer Research Program. E.D.K. and B.A.F. are Research Scholars (Pfizer and Wyland F. Leadbetter Scholars, respectively) of the American Foundation for Urologic Diseases. E.D.K. is also a past Clinical Oncology Fellow of the American Cancer Society.
Abbreviations
- TRAMP
transgenic adenocarcinoma of mouse prostate
- C2
TRAMP-C2
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- H&E
hematoxylin and eosin
References
- 1.Leach D, Krummel M, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 2.Kwon E D, Hurwitz A A, Foster B A, Madias C, Feldhaus A L, Greenberg N M, Burg M B, Allison J P. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster B A, Gingrich J R, Kwon E D, Madias C, Greenberg N M. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 4.Hurwitz A A, Yu T F-Y, Leach D R, Allison J P. Proc Natl Acad Sci USA. 1998;95:1067–1071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Elsas A, Hurwitz A A, Allison J P. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison J P. Curr Opin Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 7.Chambers C A, Krummel M F, Boitel B, Hurwitz A, Sullivan T J, Fournier S, Cassell D, Brunner M, Allison J P. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson C B, Allison J P. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg N M, DeMayo F, Finegold M J, Medina D, Tilley W D, Aspinall J O, Cunha G R, Donjacour A A, Matusik R J, Rosen J M. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamey T A, Kabalin J N, McNeal J E, Johnston I M, Freiha F, Redwine E A, Yang N. J Urol. 1989;141:1076–1083. doi: 10.1016/s0022-5347(17)41175-x. [DOI] [PubMed] [Google Scholar]
- 11.Donovan J F, Lubaroff D M, Williams R D. Probl Urol. 1990;4:490–505. [Google Scholar]
- 12.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahne, M., Rimoldi, D., Schroter, M., Romero, P., Schreier, M. & French, L. E. J. Science274, 1363–1366. [DOI] [PubMed]
- 14.North R J. J Exp Med. 1982;55:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awwad M, North R J. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 16.Mizoguchi, H., O'Shea, J. J., Longo, D. L., Loeffler, C. M., McVicar, D. W. & Ochoa, A. C. Science258, 1795–1798. [DOI] [PubMed]
- 17.Finke J H, Zea A H, Stanley J, Longo D L, Mizoguchi H, Tubbs R R, Wiltrout R H, O'Shea J J, Kudoh S, Klein E, et al. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 18.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin J L, Vivier E, Anderson P, Kiessling R. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 19.Tartour E, Latour S, Mathiot C, Thiounn N, Mosseri V, Joyeux I, D'Enghien C D, Lee R, Debre B, Fridman W H. Int J Cancer. 1995;63:205–212. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 20.Renner C, Obnesorge S, Held G, Bauer S, Jung W, Pfitzenmeier J P, Pfeundschuh M. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- 21.Alexander J P, Kudoh S, Melsop K A, Hamilton T A, Edinger M G, Tubbs R R, Sica D, Tuason L, Klein E, Bukowski R M, Finke J H. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- 22.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 23.Bouck N, Stellmach V, Hsu S C. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin C M, Salhany K E, Gee M S, LaTemple D C, Kotenko S, Ma X, Gri G, Wysocka M, Kim J E, Liu L, et al. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 25.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]