Abstract
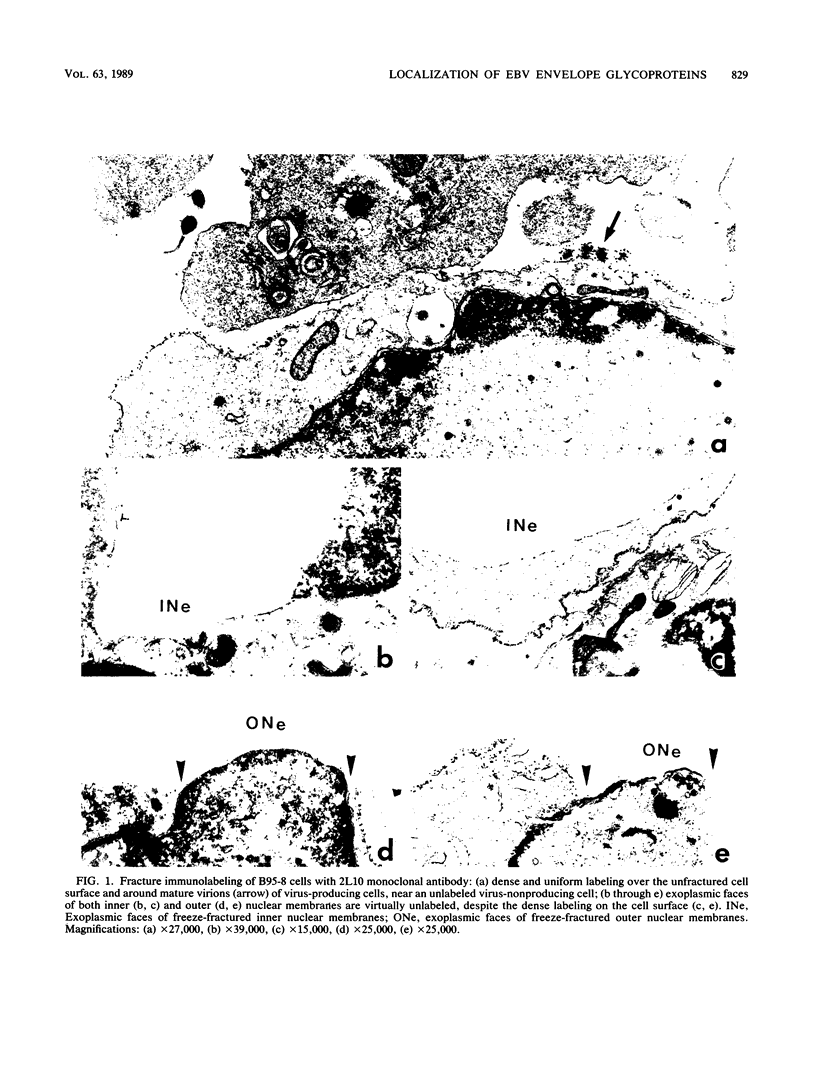
Epstein-Barr virus-producing cells were used as a model to analyze, with a fracture-immunolabel technique, the distribution, behavior on fracture, and extent of glycosylation of viral transmembrane glycoproteins at the inner nuclear membrane. Surface and fracture immunolabeling with two monoclonal antibodies directed against the carbohydrate or polypeptide portions of the major viral envelope glycoproteins gp350/220 showed the following. (i) The glycoproteins present on the inner and outer nuclear membranes were labeled only with the monoclonal antibody directed against the polypeptide chain, whereas over the surface of virus-producing cells and on mature virions the labeling was dense and uniformly distributed with both monoclonal antibodies. (ii) The glycoproteins were nonuniformly distributed only over the inner nuclear membranes; at the sites of viral budding, the glycoproteins showed a preferential partition with the protoplasmic face. Since fully glycosylated glycoproteins were not present on the nuclear membranes, our observations support the proposed model of herpesvirus maturation. The peculiar distribution and partition on fracture of the envelope glycoproteins on the inner nuclear membrane are similar to those of Sindbis virus envelope glycoproteins on the plasma membrane of infected cells. Therefore, our results suggest that inner nuclear membranes may behave like plasma membranes during viral assembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edson C. M., Thorley-Lawson D. A. Synthesis and processing of the three major envelope glycoproteins of Epstein-Barr virus. J Virol. 1983 May;46(2):547–556. doi: 10.1128/jvi.46.2.547-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., LeBlanc P. A. Modification of Epstein-Barr virus replication by tunicamycin. J Virol. 1986 Jan;57(1):117–123. doi: 10.1128/jvi.57.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. J., Smith A. R., Barker R. N., Epstein M. A. A structural investigation of the Epstein-Barr (EB) virus membrane antigen glycoprotein, gp340. J Gen Virol. 1984 Feb;65(Pt 2):397–404. doi: 10.1099/0022-1317-65-2-397. [DOI] [PubMed] [Google Scholar]
- Norrild B., Pedersen B. Effect of tunicamycin on the synthesis of herpes simplex virus type 1 glycoproteins and their expression on the cell surface. J Virol. 1982 Aug;43(2):395–402. doi: 10.1128/jvi.43.2.395-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan A., Lotti L. V., Torrisi M. R., Migliaccio G., Bonatti S. Regional distribution of Sindbis virus glycoproteins on the plasma membrane of infected baby hamster kidney cells. Exp Cell Res. 1987 Jan;168(1):53–62. doi: 10.1016/0014-4827(87)90415-0. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Henle G., Henle W. Production of antigens associated with Epstein-Barr virus in experimentally infected lymphoblastoid cell lines. J Natl Cancer Inst. 1971 Jun;46(6):1243–1250. [PubMed] [Google Scholar]
- Pinto da Silva P., Parkison C., Dwyer N. Fracture-label:O cytochemistry of freeze-fracture faces in the erythrocyte membrane. Proc Natl Acad Sci U S A. 1981 Jan;78(1):343–347. doi: 10.1073/pnas.78.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliquin L., Levine G., Shore G. C. Involvement of Golgi apparatus and a restructured nuclear envelope during biogenesis and transport of herpes simplex virus glycoproteins. J Histochem Cytochem. 1985 Sep;33(9):875–883. doi: 10.1177/33.9.2991363. [DOI] [PubMed] [Google Scholar]
- Seigneurin J. M., Vuillaume M., Lenoir G., De-Thé G. Replication of Epstein-Barr virus: ultrastructural and immunofluorescent studies of P3HR1-superinfected Raji cells. J Virol. 1977 Dec;24(3):836–845. doi: 10.1128/jvi.24.3.836-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Adams M. R., Rabin H. Glycosylation pathways of two major Epstein-Barr virus membrane antigens. Virology. 1983 May;127(1):168–176. doi: 10.1016/0042-6822(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Tanner J., Weis J., Fearon D., Whang Y., Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987 Jul 17;50(2):203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Torrisi M. R., Bonatti S. Immunocytochemical study of the partition and distribution of Sindbis virus glycoproteins in freeze-fractured membranes of infected baby hamster kidney cells. J Cell Biol. 1985 Oct;101(4):1300–1306. doi: 10.1083/jcb.101.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Da Silva P. P. T-lymphocyte heterogeneity: wheat germ agglutinin labeling of transmembrane glycoproteins. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5671–5674. doi: 10.1073/pnas.79.18.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Lotti L. V., Pavan A., Migliaccio G., Bonatti S. Free diffusion to and from the inner nuclear membrane of newly synthesized plasma membrane glycoproteins. J Cell Biol. 1987 Mar;104(3):733–737. doi: 10.1083/jcb.104.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Pinto da Silva P. Compartmentalization of intracellular membrane glycocomponents is revealed by fracture-label. J Cell Biol. 1984 Jan;98(1):29–34. doi: 10.1083/jcb.98.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A., Koide N., Klein G. Two large virion envelope glycoproteins mediate Epstein-Barr virus binding to receptor-positive cells. J Virol. 1982 Jan;41(1):286–297. doi: 10.1128/jvi.41.1.286-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva P. P., Kachar B., Torrisi M. R., Brown C., Parkison C. Freeze-fracture cytochemistry: replicas of critical point-dried cells and tissues after fracture-label. Science. 1981 Jul 10;213(4504):230–233. doi: 10.1126/science.7244630. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Parkison C., Dwyer N. Freeze-fracture cytochemistry: thin sections of cells and tissues after labeling of fractures faces. J Histochem Cytochem. 1981 Aug;29(8):917–928. doi: 10.1177/29.8.7276536. [DOI] [PubMed] [Google Scholar]
- da Silva P. P., Torrisi M. R. Freeze-fracture cytochemistry: partition of glycophorin in freeze-fractured human erythrocyte membranes. J Cell Biol. 1982 May;93(2):463–469. doi: 10.1083/jcb.93.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]





