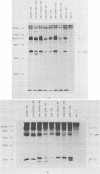
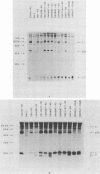
Abstract
Hybrid viral genomes were used to investigate the influence of specific polyomavirus sequences on the transforming behavior of JC virus (JCV). One set of chimeric DNAs was made by exchanging the regulatory regions between JCV and simian virus 40 (SV40) or JCV and BK virus (BKV). A second set of constructs was produced that expressed hybrid JCV-BKV T proteins under the control of either JCV or BKV regulatory signals. Transformation of Rat 2 cells with the parental and chimeric DNAs indicated that both the JCV regulatory signals and the sequence encoding the amino terminus of T protein contributed to the restricted transforming behavior of this virus. Analysis of the viral proteins in the transformed rat cells indicated that the large T antigens of JCV and BKV were less stable than their SV40 counterpart, that small t protein was produced in JCV transformants, and that the subpopulation of T antigen that forms a stable complex with cellular p53 protein was smaller in JCV-transformed cells than in SV40- or BKV-transformed cells.
Full text
PDF

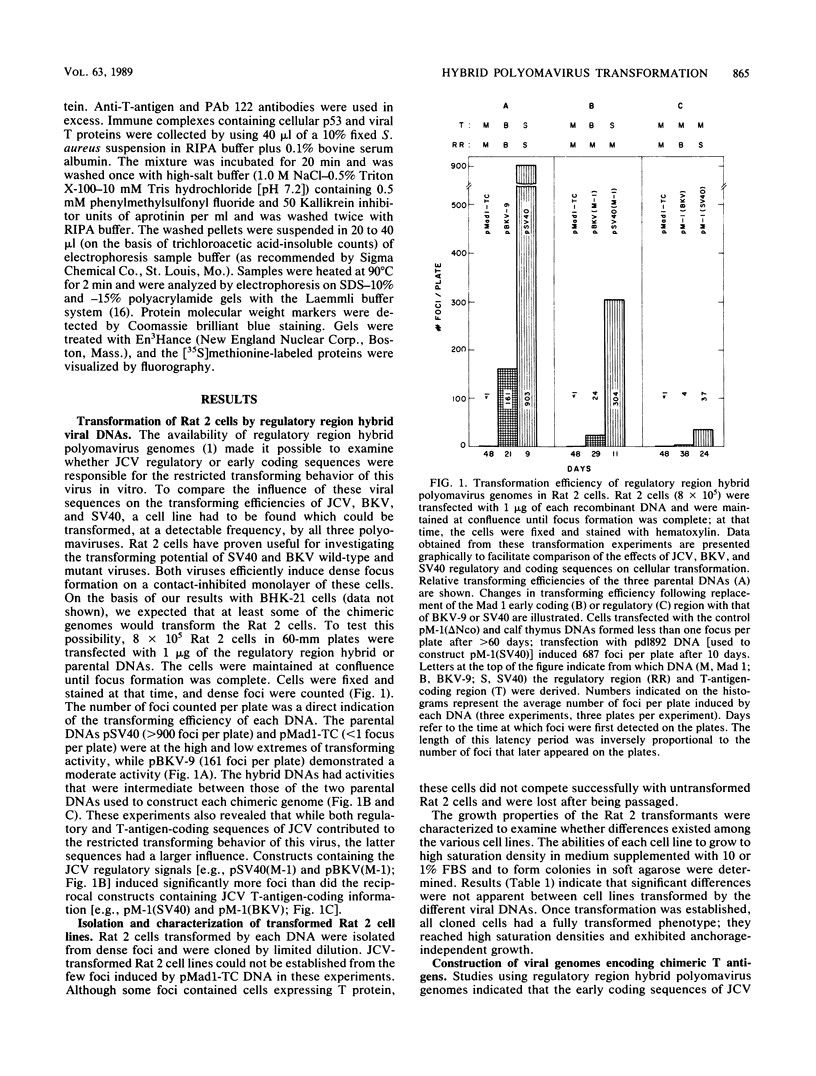

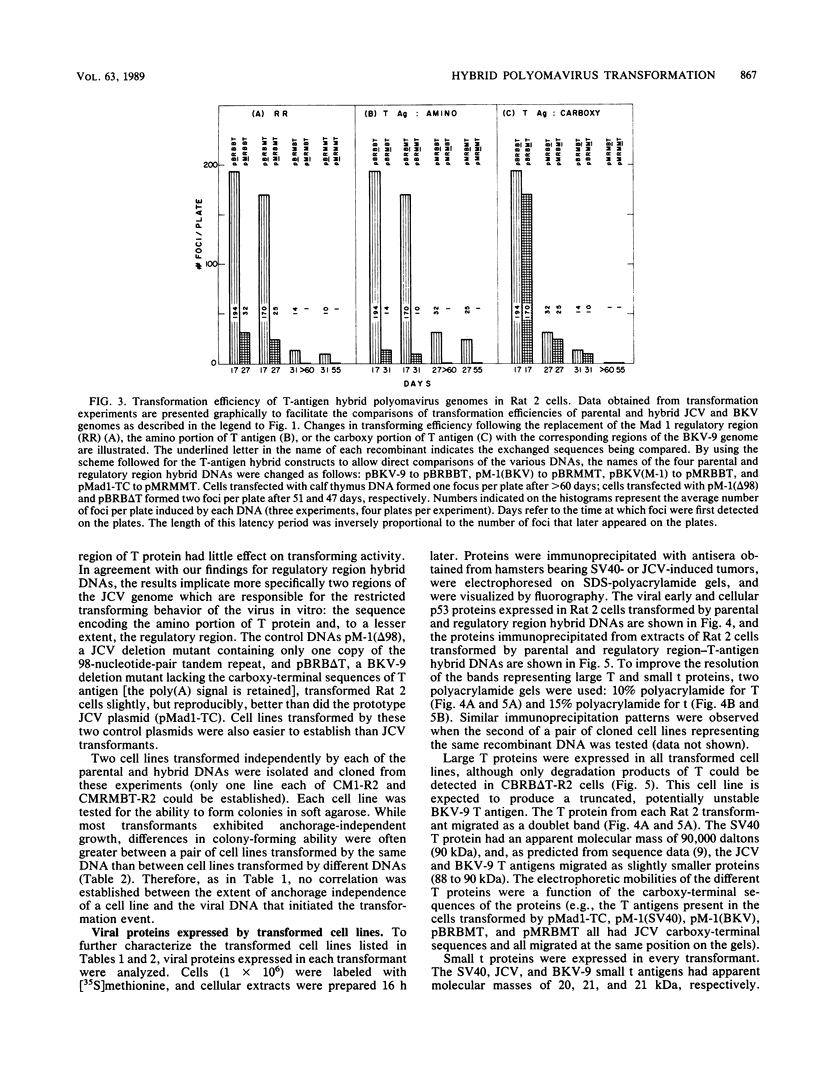

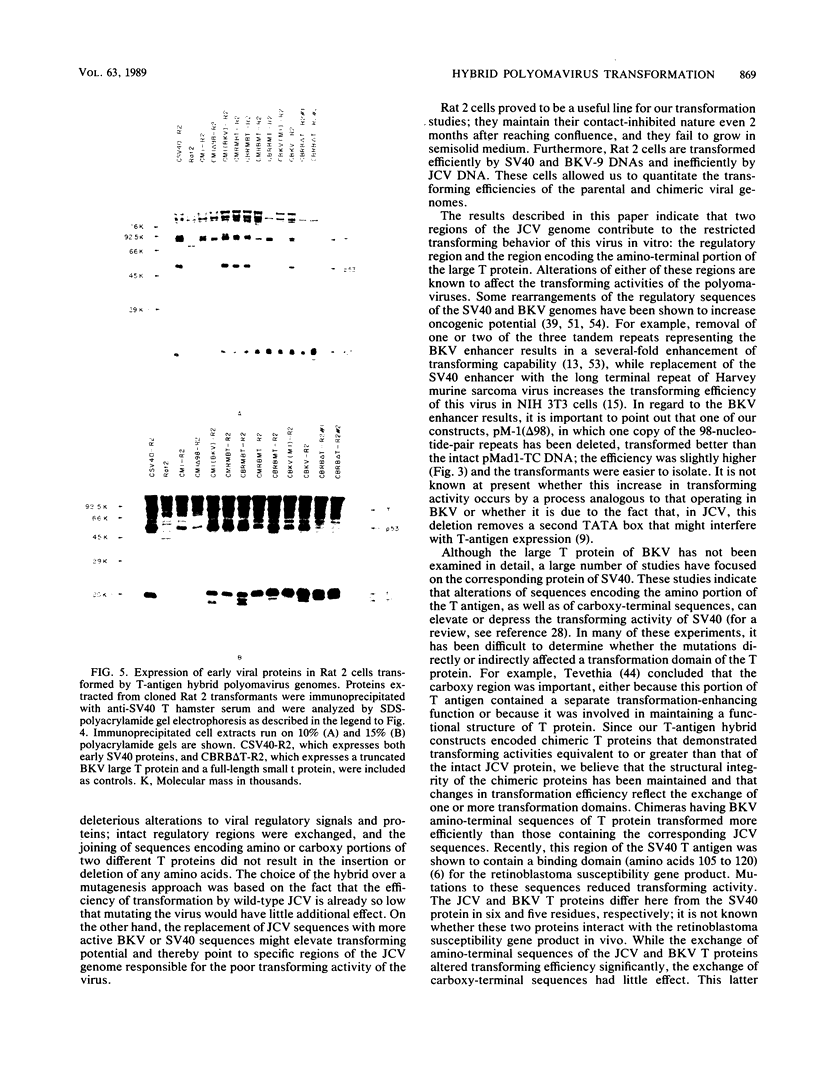



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuke W. F., Walker D. L., Peitzman L. B., Frisque R. J. Construction and characterization of hybrid polyomavirus genomes. J Virol. 1986 Dec;60(3):960–971. doi: 10.1128/jvi.60.3.960-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Murphy D., Lovett M., Rigby P. W. A fragment of the SV40 large T-antigen gene transforms. Nature. 1982 Sep 2;299(5878):59–61. doi: 10.1038/299059a0. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Regulatory sequences and virus-cell interactions of JC virus. Prog Clin Biol Res. 1983;105:41–59. [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Walker D. L. Transformation of primary hamster brain cells with JC virus and its DNA. J Virol. 1980 Jul;35(1):265–269. doi: 10.1128/jvi.35.1.265-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Oya Y., Yogo Y. Enhancement of the transforming capacity of BK virus by partial deletion of the 68-base-pair tandem repeats. J Virol. 1985 Sep;55(3):867–869. doi: 10.1128/jvi.55.3.867-869.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Botchan M. Enhanced transformation by a simian virus 40 recombinant virus containing a Harvey murine sarcoma virus long terminal repeat. Mol Cell Biol. 1983 Mar;3(3):325–339. doi: 10.1128/mcb.3.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt A., Chen S., Blanck G., George D., Pollack R. E. Two integrated partial repeats of simian virus 40 together code for a super-T antigen. Mol Cell Biol. 1985 Apr;5(4):742–750. doi: 10.1128/mcb.5.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Major E. O. JC virus T protein expression in owl monkey tumor cell lines. Prog Clin Biol Res. 1983;105:289–298. [PubMed] [Google Scholar]
- Major E. O., Mourrain P., Cummins C. JC virus-induced owl monkey glioblastoma cells in culture: biological properties associated with the viral early gene product. Virology. 1984 Jul 30;136(2):359–367. doi: 10.1016/0042-6822(84)90172-7. [DOI] [PubMed] [Google Scholar]
- Mandl C., Walker D. L., Frisque R. J. Derivation and characterization of POJ cells, transformed human fetal glial cells that retain their permissivity for JC virus. J Virol. 1987 Mar;61(3):755–763. doi: 10.1128/jvi.61.3.755-763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., King D. M., Slauch J. M., Frisque R. J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985 Jan;53(1):306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Eliyahu D., Oren M. Overproduction of protein p53 contributes to simian virus 40-mediated transformation. Mol Cell Biol. 1986 Oct;6(10):3531–3536. doi: 10.1128/mcb.6.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole S. E., Gannon J. V., Ford M. J., Lane D. P. Structure and function of SV40 large-T antigen. Philos Trans R Soc Lond B Biol Sci. 1987 Dec 15;317(1187):455–469. doi: 10.1098/rstb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Ohsumi S., Motoi M., Ogawa K. Induction of undifferentiated tumors by JC virus in the cerebrum of rats. Acta Pathol Jpn. 1986 Jun;36(6):815–825. doi: 10.1111/j.1440-1827.1986.tb03116.x. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Virologic and serologic studies of progressive multifocal leukoencephalopathy. Prog Clin Biol Res. 1983;105:107–117. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Hodach A. E., Chou S. M. JC Papovavirus in progressive multifocal leukoencephalopathy. J Infect Dis. 1976 Jun;133(6):686–690. doi: 10.1093/infdis/133.6.686. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Varakis J. N. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977 Mar;37(3):718–720. [PubMed] [Google Scholar]
- Pipas J. M. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985 May;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gluzman Y., Winocour E. Cellular and cell-free synthesis of simian virus 40 T-antigens in permissive and transformed cells. J Virol. 1978 Feb;25(2):587–595. doi: 10.1128/jvi.25.2.587-595.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieth K. G., Di Chiro G., London W. T., Sever J. L., Houff S. A., Kornblith P. L., McKeever P. E., Buonomo C., Padgett B. L., Walker D. L. Experimental glioma in primates: a computed tomography model. J Comput Assist Tomogr. 1980 Jun;4(3):285–290. doi: 10.1097/00004728-198006000-00001. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Gurney E. G., Danna K. J. Stabilization of the 53,000-dalton nonviral tumor antigen is not required for transformation by simian virus 40. Mol Cell Biol. 1983 Feb;3(2):290–296. doi: 10.1128/mcb.3.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Danna K. J. Less than 40% of the simian virus 40 large T-antigen-coding sequence is required for transformation. Mol Cell Biol. 1984 Aug;4(8):1661–1663. doi: 10.1128/mcb.4.8.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S., Yamaguchi N. Two classes of transformation-deficient, immortalization-positive simian virus 40 mutants constructed by making three-base insertions in the T antigen gene. J Virol. 1984 Dec;52(3):884–891. doi: 10.1128/jvi.52.3.884-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wright J. H., Gurney E. G. Characterization of simian virus 40 large T antigen by using different monoclonal antibodies: T-p53 complexes are preferentially ATPase active and adenylylated. J Virol. 1988 Mar;62(3):1028–1037. doi: 10.1128/jvi.62.3.1028-1037.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Anderson R. W., Tevethia S. S., Simmons D., Feunteun J., Cole C. Influence of amino acids encoded in the 3' open reading frame of the SV40 early region on transformation and antigenicity of large T antigen. Virology. 1986 Apr 30;150(2):361–372. doi: 10.1016/0042-6822(86)90301-6. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J. Immortalization of primary mouse embryo fibroblasts with SV40 virions, viral DNA, and a subgenomic DNA fragment in a quantitative assay. Virology. 1984 Sep;137(2):414–421. doi: 10.1016/0042-6822(84)90234-4. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Tordaro G. J., Green H. An assay for cellular transformation by SV40. Virology. 1964 May;23(1):117–119. doi: 10.1016/s0042-6822(64)80018-0. [DOI] [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Soeda E., Uchida S., Yoshiike K. DNA rearrangement affecting expression of the BK virus transforming gene. J Virol. 1984 Jul;51(1):1–6. doi: 10.1128/jvi.51.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Decreasing the number of 68-base-pair tandem repeats in the BK virus transcriptional control region reduces plaque size and enhances transforming capacity. J Virol. 1985 Sep;55(3):823–825. doi: 10.1128/jvi.55.3.823-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Olson L., Tyndall C., Schaffner W. Transcriptional 'enhancers' from SV40 and polyoma virus show a cell type preference. Nucleic Acids Res. 1982 Dec 20;10(24):7965–7976. doi: 10.1093/nar/10.24.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]