Abstract
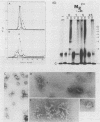
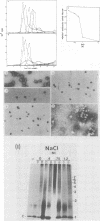
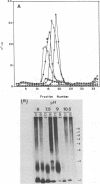
Purified intact Sindbis virus nucleocapsids were treated at different pH values or with various concentrations of divalent cations, cation chelators, salt, or formamide. The resulting structures were examined by velocity sedimentation, electron microscopy, and protein-protein cross-linking. Changes in each of the test conditions led to alterations in the sedimentation profile of treated nucleocapsids. Appropriate concentrations of formamide or divalent cations generated beaded strandlike structures similar in morphology to those generated from adenovirus cores and nucleosomes. The capsid protein and RNA remained associated with each other at NaCl concentrations less than or equal to 1 M or after treatment of the structures with alkaline pH up to and including pH 10.7. Protein and RNA were dissociated by salt concentrations of greater than 1 M, suggesting that the arginine-rich, amino-terminal portion of the capsid protein is responsible for binding the RNA. Protein-protein cross-linking also indicated that the capsid proteins remained associated in small aggregates under some of the conditions that caused dissociation of the nucleocapsid and suggested the presence of more than one type of protein-protein interaction in the nucleocapsids. Collectively, these data suggest that, like histones and adenovirus core proteins, the Sindbis virus capsid protein serves to package segments of the genome into nucleoprotein beads which are capable of interacting with each other to form the nucleocapsid structure.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKINRIMISI E. O., BONNER J., TSO P. O. BINDING OF BASIC PROTEINS TO DNA. J Mol Biol. 1965 Jan;11:128–136. doi: 10.1016/s0022-2836(65)80178-4. [DOI] [PubMed] [Google Scholar]
- Acheson N. H., Tamm I. Ribonuclease sensitivity of Semliki Forest virus nucleocapsids. J Virol. 1970 Jun;5(6):714–717. doi: 10.1128/jvi.5.6.714-717.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J. B., Hiebert E., Rees M. W., Markham R. Properties of cowpea chlorotic mottle virus, its protein and nucleic acid. Virology. 1968 Feb;34(2):224–239. doi: 10.1016/0042-6822(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Weber J. Virion core-like organization of intranuclear adenovirus chromatin late in infection. Virology. 1980 Nov;107(1):306–310. doi: 10.1016/0042-6822(80)90297-4. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Formation of Sindbis virus capsid protein in mammalian cell-free extracts programmed with viral messenger RNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1843–1847. doi: 10.1073/pnas.71.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Brown D. T. Organization of the Sindbis virus nucleocapsid as revealed by bifunctional cross-linking agents. J Mol Biol. 1987 May 20;195(2):359–371. doi: 10.1016/0022-2836(87)90657-7. [DOI] [PubMed] [Google Scholar]
- Coombs K., Brown D. T. Topological organization of Sindbis virus capsid protein in isolated nucleocapsids. Virus Res. 1987 Apr;7(2):131–149. doi: 10.1016/0168-1702(87)90075-x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Enzmann P. J., Weiland F. Studies on the morphology of alphaviruses. Virology. 1979 Jun;95(2):501–510. doi: 10.1016/0042-6822(79)90504-x. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Conformational equilibria in -and -chymotrypsin. The energetics and importance of the salt bridge. J Mol Biol. 1972 Mar 14;64(2):497–509. doi: 10.1016/0022-2836(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- GIANNONI G., PEACOCKE A. R. Thymus deoxyribonucleoprotein. III. Sedimentation behaviour. Biochim Biophys Acta. 1963 Feb 26;68:157–166. doi: 10.1016/0006-3002(63)90131-8. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Virus structure: high-resolution perspectives. Adv Virus Res. 1983;28:175–240. doi: 10.1016/s0065-3527(08)60724-1. [DOI] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Horzinek M., Mussgay M. Studies on the nucleocapsid structure of a group A arbovirus. J Virol. 1969 Oct;4(4):514–520. doi: 10.1128/jvi.4.4.514-520.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Sung M. T. Isolation and characterization of an extremely basic protein from adenovirus type 5. J Virol. 1976 Mar;17(3):924–934. doi: 10.1128/jvi.17.3.924-934.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- INCARDONA N. L., KAESBERG P. A PH-INDUCED STRUCTURAL CHANGE IN BROMEGRASS MOSAIC VIRUS. Biophys J. 1964 Jan;4:11–21. doi: 10.1016/s0006-3495(64)86766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Jacrot B. Studies on the assembly of a spherical plant virus. II. The mechanism of protein aggregation and virus swelling. J Mol Biol. 1975 Jul 5;95(3):433–446. doi: 10.1016/0022-2836(75)90201-6. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- Kaper J. M. Arrangement and identification of simple isometric viruses according to their dominating stabilizing interactions. Virology. 1973 Sep;55(1):299–304. doi: 10.1016/s0042-6822(73)81035-9. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Properties of Semliki Forest virus nucleocapsid. 1. Sensitivity to pancreatic ribonuclease. Virology. 1971 Jan;43(1):291–299. doi: 10.1016/0042-6822(71)90246-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine R., Söderlund H., Renkonen O. Chemical composition of Semliki forest virus. Intervirology. 1973;1(2):110–118. doi: 10.1159/000148837. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of poliovirus. II. Evidence for the self-assembly of 14 S particles into empty capsids. Virology. 1971 May;44(2):307–316. doi: 10.1016/0042-6822(71)90262-5. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Philipson L. Internal basic proteins in adenovirus. Virology. 1968 Nov;36(3):508–511. doi: 10.1016/0042-6822(68)90178-5. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Differences between poliovirus empty capsids formed in vivo and those formed in vitro: a role for the morphopoietic factor. J Virol. 1981 Oct;40(1):173–183. doi: 10.1128/jvi.40.1.173-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig M., Orrell S. A. A technique for the electron microscopy of protein-free particle suspensions by the negative staining method. J Ultrastruct Res. 1970 Jul;32(1):107–117. doi: 10.1016/s0022-5320(70)80040-5. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Association of sindbis virion glycoproteins and their precursors. J Mol Biol. 1982 Jan 15;154(2):325–348. doi: 10.1016/0022-2836(82)90067-5. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson G., Kaesberg P. An artificial top component of R17 bacteriophage. J Mol Biol. 1970 Jan 14;47(1):87–91. doi: 10.1016/0022-2836(70)90403-1. [DOI] [PubMed] [Google Scholar]
- Scheefers H., Scheefers-Borchel U., Edwards J., Brown D. T. Distribution of virus structural proteins and protein-protein interactions in plasma membrane of baby hamster kidney cells infected with Sindbis or vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7277–7281. doi: 10.1073/pnas.77.12.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V. Immune lysis of Sindbis virus. Virology. 1975 Aug;66(2):620–624. doi: 10.1016/0042-6822(75)90235-4. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H. Kinetics of formation of the Semliki Forest virus nucleocapsid. Intervirology. 1973;1(5-6):354–361. doi: 10.1159/000148864. [DOI] [PubMed] [Google Scholar]
- Söderlund H., von Bonsdorff C. H., Ulmanen I. Comparison of the structural properties of Sindbis and Semliki forest virus nucleocapsids. J Gen Virol. 1979 Oct;45(1):15–26. doi: 10.1099/0022-1317-45-1-15. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Rogers A. E., Flint S. J. The structure of nucleoprotein cores released from adenovirions. Nucleic Acids Res. 1983 Jan 25;11(2):441–460. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]