Abstract
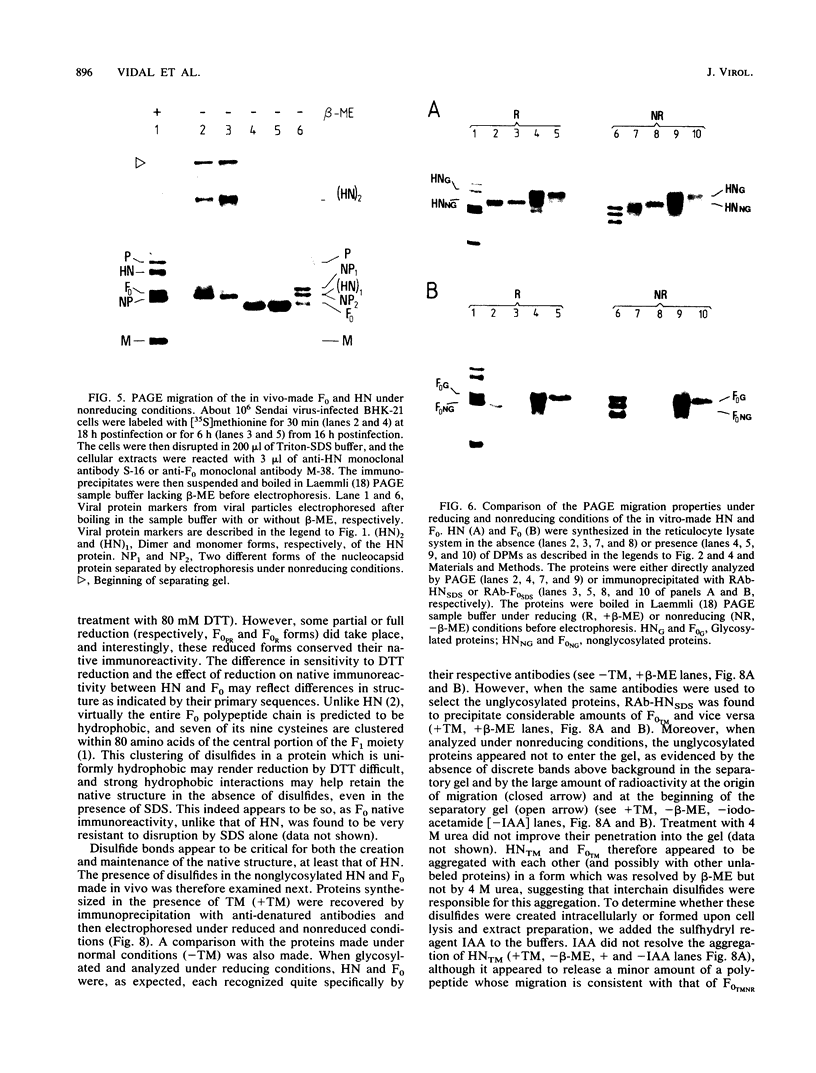
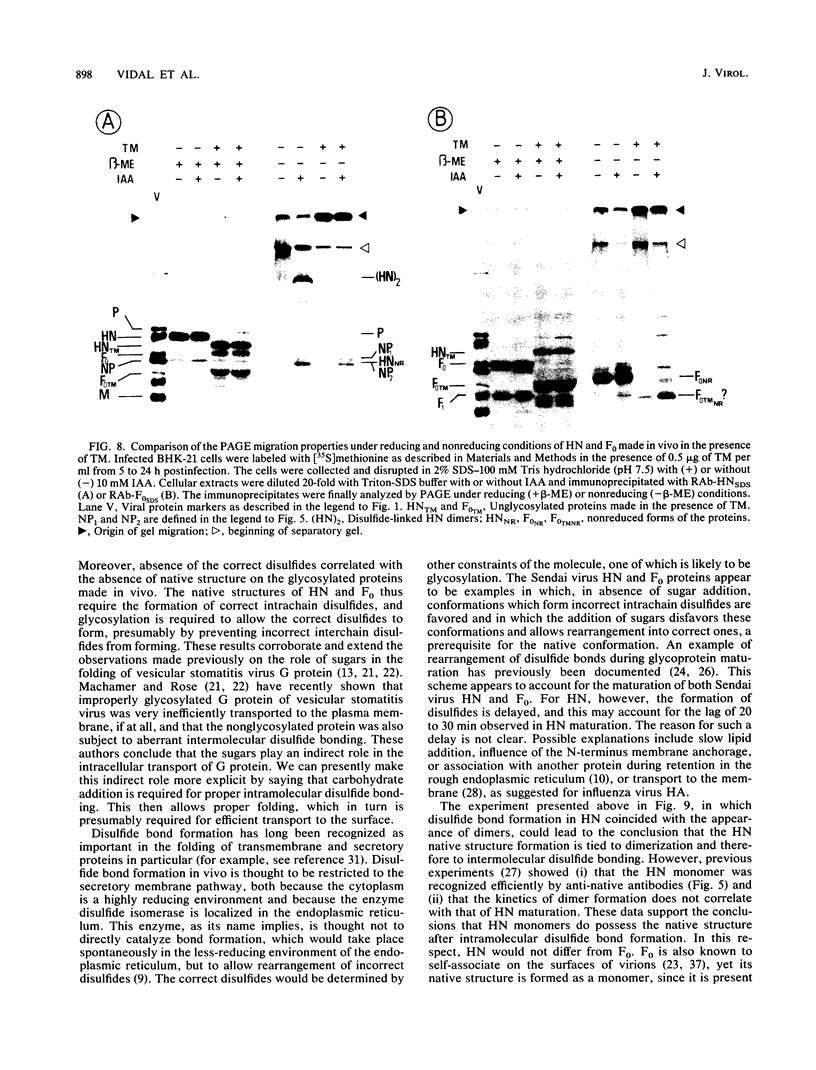
The role of glycosylation and of disulfide bonds in the formation of the native structure of the Sendai virus hemagglutinin-neuraminidase (HN) and fusion (F0) glycoproteins was studied. In contrast to the HN and F0 synthesized in vivo, the proteins made from pSP6 transcripts in reticulocyte lysates, whether glycosylated or not, were not recognized by monoclonal antibodies or polyclonal rabbit sera raised against the native proteins; they efficiently reacted only with rabbit antisera raised against the reduced sodium dodecyl sulfate-denatured proteins. These in vitro-made proteins, however, did not contain disulfide bonds. The proteins made in vivo in the presence of tunicamycin, which were also not recognized by the anti-native protein antibodies, did contain disulfide bonds, but they were mainly incorrect interchain disulfide bonds. Moreover, while F0 acquired proper disulfide bonds as soon as it was synthesized under normal conditions in vivo, the disulfides were formed in HN only after a lag of 10 to 30 min. This lag coincides with the delay observed in HN native structure formation. We therefore conclude that the maturation of the HN and F0 proteins depends on the formation of proper intramolecular disulfide bonds, which in turn depends on the previous addition of high-mannose sugars.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Rose K., Simona M. G., Roux L., Giorgi C., Kolakofsky D. Analysis of the Sendai virus M gene and protein. J Virol. 1984 Nov;52(2):656–663. doi: 10.1128/jvi.52.2.656-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J. J., Steckman M. L. Comparison of exogenous energy sources for in vitro maintenance of follicle cell-free Xenopus laevis oocytes. In Vitro. 1976 Mar;12(3):173–179. doi: 10.1007/BF02796439. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Kornfeld S., Schlesinger S. The effect of oligosaccharide chains of different sizes on the maturation and physical properties of the G protein of vesicular stomatitis virus. J Biol Chem. 1981 Jan 10;256(1):456–462. [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Gianni A. M., Comi P., Ottolenghi S., Rungger D. Translational control of globin synthesis by haemin in Xenopus oocytes. Nat New Biol. 1973 Nov 28;246(152):99–102. doi: 10.1038/newbio246099a0. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Schulman J. L., Young J. F., Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983 Apr 15;126(1):106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Influence of new glycosylation sites on expression of the vesicular stomatitis virus G protein at the plasma membrane. J Biol Chem. 1988 Apr 25;263(12):5948–5954. [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnes L. W., Semerjian A., Morrison T. Conformational changes in Newcastle disease virus fusion glycoprotein during intracellular transport. J Virol. 1985 Nov;56(2):341–348. doi: 10.1128/jvi.56.2.341-348.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Peeples M. E., McGinnes L. W. Conformational change in a viral glycoprotein during maturation due to disulfide bond disruption. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1020–1024. doi: 10.1073/pnas.84.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet G., Portner A., Roux L. Drastic immunoreactivity changes between the immature and mature forms of the Sendai virus HN and F0 glycoproteins. J Virol. 1986 Jul;59(1):132–141. doi: 10.1128/jvi.59.1.132-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mücke K., Scholtissek C. Extragenic and intragenic suppression of a transport mutation in the hemagglutinin gene of an influenza A virus as revealed by backcross and sequence determination. Virology. 1987 May;158(1):112–117. doi: 10.1016/0042-6822(87)90243-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Glycopeptide components of influenza viral glycoproteins. Virology. 1978 May 15;86(2):432–442. doi: 10.1016/0042-6822(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Homma M., Compans R. W. Effect of tunicamycin on the replication of Sendai virus. Virology. 1982 Jun;119(2):474–487. doi: 10.1016/0042-6822(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Pace C. N., Creighton T. E. The disulphide folding pathway of ribonuclease T1. J Mol Biol. 1986 Apr 5;188(3):477–486. doi: 10.1016/0022-2836(86)90169-5. [DOI] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Portner A. The HN glycoprotein of Sendai virus: analysis of site(s) involved in hemagglutinating and neuraminidase activities. Virology. 1981 Dec;115(2):375–384. doi: 10.1016/0042-6822(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Roux L., Beffy P., Portner A. Restriction of cell surface expression of Sendai virus hemagglutinin-neuraminidase glycoprotein correlates with its higher instability in persistently and standard plus defective interfering virus infected BHK-21 cells. Virology. 1984 Oct 15;138(1):118–128. doi: 10.1016/0042-6822(84)90152-1. [DOI] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechoy O., Philippot J. R., Bienvenue A. F protein-F protein interaction within the Sendai virus identified by native bonding or chemical cross-linking. J Biol Chem. 1987 Aug 25;262(24):11519–11523. [PubMed] [Google Scholar]
- Stallcup K. C., Fields B. N. The replication of measles virus in the presence of tunicamycin. Virology. 1981 Jan 30;108(2):391–404. doi: 10.1016/0042-6822(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Yellen A., Bächi T. Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell. 1988 Mar 25;52(6):843–852. doi: 10.1016/0092-8674(88)90426-6. [DOI] [PubMed] [Google Scholar]












