Abstract
The relationship between glucocorticoids (GCs) and memory is complex, in that memory impairments can occur in response to manipulations that either increase or decrease GC levels. We investigated this issue by assessing the relationship between serum corticosterone (the primary rodent GC) and memory in rats trained in the radial arm water maze, a hippocampus-dependent spatial memory task. Each day, rats learned a new location of the hidden escape platform and then 30 min later their memory of the location of the platform was tested. Under control conditions, well-trained rats had excellent spatial memory and moderately elevated corticosterone levels (~26 μg/dl versus a baseline of ~2 μg/dl). Their memory was impaired when corticosterone levels were either reduced by metyrapone (a corticosterone synthesis inhibitor) or increased by acute stress (predator exposure), forming an overall U-shaped relationship between corticosterone levels and memory. We then addressed whether there was a causal relationship between elevated corticosterone levels and impaired memory. If elevated corticosterone levels were a sufficient condition to impair memory, then exogenously administered corticosterone, alone, should have impaired performance. However, we found that spatial memory was not impaired in corticosterone-injected rats that were not exposed to the cat. This work demonstrates that an intermediate level of corticosterone correlated with optimal memory, and either a decrease or an increase in corticosterone levels, in conjunction with strong emotionality, impaired spatial memory. These findings indicate that fear-provoking conditions, which are known to engage the amygdala, interact with stress levels of corticosterone to influence hippocampal functioning.
Keywords: Glucocorticoids; Predator Stress; Hippocampus; Metyrapone; Water Maze; Amygdala, Rat
INTRODUCTION
Understanding how emotion affects memory is a challenge because of the complexity of stress-memory interactions. Whereas intense emotional experiences can generate vivid memories that can last for years after the original experience (Bremner and Vermetten 2001; Green, 2003), strong emotional experiences can also impair memory (Loftus and Burns 1982; Bower and Sivers 1998; Yovell et al., 2003; Markowitsch, 2003). Recent research has provided insight into the neurobiological basis of the complex interactions between stress and memory. Studies have shown that the hippocampus, a temporal lobe structure which is important for memory formation, is strongly affected by stress (Lupien and McEwen 1997; Nadel and Jacobs 1998; Diamond et al., 2001; Kim and Diamond 2002). For example, we have shown that stress impairs hippocampus-dependent memory in rats trained to remember either the location of food (Diamond et al., 1996) or a hidden platform in a water maze (Diamond et al., 1999; Woodson et al., 2003; Diamond et al., 2004a; Sandi et al., 2005). These findings are consistent with other studies in rodents and people demonstrating the susceptibility of hippocampus-dependent memory and synaptic plasticity, i.e., longterm potentiation (LTP), to be impaired by acute stress or increased GC levels, i.e., corticosterone in the rat or cortisol in people (see Kim and Diamond 2002; Diamond et al., 2004a for reviews).
While it is evident that stress-induced increases in corticosterone can be detrimental to hippocampal functioning, it is perhaps paradoxical that studies have also shown that manipulations which produce decreases in corticosterone levels also impair cognitive performance (Loscertales et al., 1997; Pugh et al., 1997; Conrad et al., 1999; Roozendaal, 2000; McGaugh and Roozendaal 2002). One explanation for this paradoxical finding is that there is a U-shaped relationship between corticosterone and both memory (Lupien and McEwen 1997) and hippocampal plasticity (Diamond et al., 1992; Kerr et al., 1994). However, the existence of a U-shaped function between stress or corticosterone and memory is based largely on a composite of findings from different training paradigms and species (as reviewed in Lupien and McEwen 1997; Lupien et al., 2005), but only rarely has a complete U-shaped function been demonstrated in individual studies (Yerkes and Dodson 1908; Broadhurst, 1957; Stennett, 1957; Watters et al., 1997; Lupien et al., 1999; Conrad et al., 1999).
The infrequently observed U-shaped function between corticosterone and memory is only one level of complexity in stress effects on memory. It has also been shown that, under some conditions, stress effects on hippocampal LTP and memory can be blocked without there being any reduction in the stress-evoked increase in corticosterone levels. For example, suppression of amygdala functioning (Kim et al., 2001; Kim et al., 2005) or administration of an antidepressant (tianeptine) (Shakesby et al., 2002) can block the effects of stress on spatial memory and hippocampal LTP without affecting the stress-induced increase in corticosterone levels. These findings indicate that elevated corticosterone levels, alone, may not be sufficient to impair memory.
The aim of this series of experiments was to study the relationship between corticosterone and hippocampus-dependent memory. We have found previously that acute exposure of rats to a fear-provoking experience (inescapable exposure to an unfamiliar environment or to a cat) impaired their spatial memory (Diamond et al., 1996; Diamond et al., 1999; Woodson et al., 2003; Diamond et al., 2004a; Diamond et al., 2004b; Sandi et al., 2005). We hypothesized here that if the surge in corticosterone levels that occurred during cat exposure was necessary for stress to impair memory, then metyrapone, a corticosterone synthesis blocker, should block the stress-induced impairment of spatial memory. In addition, if elevated corticosterone levels were sufficient to produce a memory impairment, then exogenous administration of corticosterone, to otherwise non-stressed rats, should mimic the memory impairments produced by predator stress. Therefore, the goal of this work was to provide a comprehensive analysis of the relationship between behaviorally and pharmacologically manipulated corticosterone levels and spatial memory in water maze-trained rats.
MATERIALS AND METHODS
Animals and Water Maze Characteristics
Adult male Harlan Sprague-Dawley rats (300g) were given one week to acclimate to the vivarium 12:12 light/dark cycle (lights on at 6AM) with food and water available ad libitum. Methodological procedures involved in training rodents and illustrations of the radial arm water maze (RAWM) have been provided in previous studies (Diamond et al., 1999; Park et al., 2001). Training took place in a tank of water (21°C) with internal walls which produced 6 swim arms that radiated out of an open central area. The arm that contained the hidden escape platform, located 1 cm below the surface of the water, is referred to as the goal arm.
Daily Training Procedures
Each day the rats were brought into the laboratory at about 9 AM and 1 hour later water maze testing began. At the start of a trial a rat was placed in the water, facing the center of the maze, in one of the arms that didn't contain the platform (start arm). The rat then swam out of the start arm into the open central area and then entered arms in search of the escape platform. Entries into arms other than the goal arm were errors. With each error the rat was returned to the start arm, as described previously (Diamond et al., 1999).
Rats were given up to 2 min per trial to find the platform and then they were allowed to remain on it for 30 seconds. In each daily training session, rats were given 4 sequential trials to learn where the platform was located (acquisition phase). The start arm was different on each trial. Well-trained rats completed the 4 trials of the acquisition phase in about 3 minutes. At the end of the fourth trial the rats were removed from the maze and returned to their home cages. Thirty min later they were returned to the maze for a fifth trial. The time between the fourth and fifth trials was the delay period, and the fifth trial was the memory test trial.
The hidden platform was always located in the same arm on each of the 5 trials within a day. Across days, the platform was pseudorandomly located in any one of the six arms, with the exception that it was never in the same arm on two consecutive days. This procedure, therefore, tested hippocampus-dependent (working) memory because the rats had to learn (on Trials 1–4), and then remember (tested 30 min later on Trial 5), which arm contained the hidden platform on that day. Previous studies have shown that performance on this task was impaired in rats that were chronically (Park et al., 2001; Gerges et al., 2004) or acutely (Diamond et al., 1999; Woodson et al., 2003; Sandi et al., 2005) stressed, or that had hippocampal damage (Diamond et al., 1999).
All stress manipulations and corticosterone injections (described below) took place during the delay period, i.e., after the 4 learning trials were completed and before the fifth (memory test) trial. Therefore, the data analysis focused on how the stress or corticosterone manipulations affected memory performance on Trial 5.
Performance Criterion
Evidence of efficient within-day learning and memory was quantified using our standard performance criterion (Diamond et al., 1999; Park et al., 2001). To meet the criterion a rat could commit a total of no more than 1 error on the three memory test trials across any three consecutive days of training. The third day of the series in which a rat satisfied the 3-day criterion period was its day to criterion (DTC). This stringent performance criterion ensured that the stress and drug manipulations were administered only to rats that had exhibited excellent spatial memory for three consecutive days under control conditions.
Stress and Drug Injection Procedures
The rats were given an injection of the vehicle twice early in training (on Days 4 and 5) to acclimate them to the injection procedures. All stress and drug manipulations were carried out on well-trained rats on the two days after they reached their DTC. The same manipulation (drug or stress) was conducted on each of the two post-DTC days. The numbers of errors committed by the rats on the two days of post-DTC testing were averaged to obtain a single mean value for each animal.
Metyrapone (50 or 75 mg/kg, ip) or an equivalent volume of vehicle (1 ml/kg, ip) was injected 30 minutes before Trial 1 and corticosterone (either 5 or 1 mg/kg, sc) was injected at the start of the 30 min delay period. The two doses of metyrapone produced statistically equivalent effects on corticosterone levels and memory (all t-tests, p > 0.1). Therefore, to increase the power of the statistical tests, data from rats injected with either of the two doses of metyrapone were combined. During stress testing each rat remained in a Plexiglas container with ventilation holes next to the cat (Diamond et al., 1999).
Drugs
Metyrapone (Aldrich Pharmaceuticals) was dissolved in polyethylene glycol (PEG) and then diluted with physiological saline to a final PEG concentration of 30%. Corticosterone (Sigma) was dissolved in 95% alcohol and then diluted with physiological saline to a final concentration of 1 or 5 mg/ml in 10% alcohol.
Blood Sampling
General Procedure
Animals were placed in a restrainer and within 2 min a 0.5 ml blood sample was obtained from a cut on the tip of the tail. The serum was extracted and stored at −70°C until it was assayed for corticosterone by radioimmunoassay (ICN Pharmaceuticals).
Laboratory Baseline Blood Sampling Procedures
One group was used for the sole purpose of measuring laboratory baseline corticosterone levels in well-trained rats. All rats in this group were first given at least one week of daily water maze training. Then they were brought to the laboratory, where they remained undisturbed for one hour, followed by blood sampling. These rats, therefore, provided a measure of corticosterone levels in well-trained rats that were brought to the laboratory following standard daily procedures, but on the day of blood sampling they were not placed in the water.
Post-Memory Testing Blood Sampling Procedures
All rats in the remaining groups provided data for both, spatial memory and corticosterone levels. That is, all rats in the experimental groups were trained to their DTC, and then on the next two days they were tested under control, stress and/or drug conditions. Blood samples were obtained from rats in these groups immediately after the memory test trial on the second day after they reached their DTC.
Summary Timeline
Across Day Timeline
All rats were given daily training until they reached their DTC and then all experimental or control manipulations took place on the next two days. Therefore, all data presented in this work are from well-trained rats that had exhibited a high degree of accuracy in their within-day spatial learning and memory during preliminary training.
Within-Day Timeline
Metyrapone or vehicle was injected 30 min before the start of the acquisition phase on each of the two post-DTC training days. The 4 trials of the acquisition phase were completed in approximately 3 minutes, and then the rats were placed either in their home cages (MET- or VEH-No Stress) or with a cat (Met- or Vehicle-Stress) for 30 min. At the end of the delay period the rats were given the memory test trial. Corticosterone injections were given at the start of the 30 min delay period followed by memory testing 30 min later. The groups administered both, metyrapone and corticosterone, received the metyrapone 30 min before Trial 1 and corticosterone at the start of the delay period (immediately after Trial 4), followed by 30 min of exposure to either the cat (MET-corticosterone-Stress) or their home cage (MET-corticosterone-No Stress).
Statistical Tests
Data were analyzed with a mixed design repeated measures Analysis of Variance (ANOVA) with post-hoc Student-Newman-Keuls (SNK) tests. Non-parametric tests (Kruskal-Wallis ANOVA on ranks and Dunn's post-hoc test) were performed when appropriate. Group data are presented as the mean (± SEM) in Figures 1, 2 and 3 (top) and 4, and as the median (± interquartile range) in Figure 3 (bottom). P values < 0.05 were considered significant.
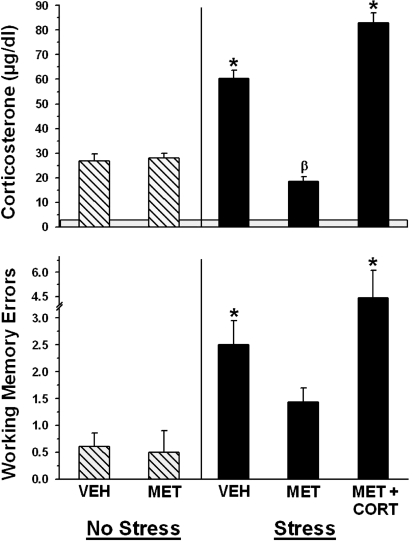
FIGURE 1.
Effects of metyrapone, cat exposure and/or corticosterone injection on corticosterone levels (top) and spatial memory errors (bottom). Top: The shaded region indicates the mean (2.5 ug/dl) of the laboratory baseline corticosterone levels. VEH and MET on the left (Home Cage) side indicates that these two groups received either vehicle or metyrapone 30 min prior to Trial 1, and then they spent the delay period between Trials 4 and 5 in their home cages. VEH, MET or MET + CORT on the right (Stress) side indicates that these three groups received vehicle, metyrapone or metyrapone and corticosterone injection, and these rats spent the delay period with the cat (Stress). The ∗ indicates that the VEH and MET+CORT groups had significantly greater corticosterone levels than all of the other groups. The β indicates that the MET – Stress group had significantly lower corticosterone than all of the other groups trained in the water maze. Bottom: Spatial memory errors (Trial 5 performance) for the 5 groups given the treatments described above. The ∗ indicates that the VEH and MET + CORT Stress groups committed significantly more errors than the two Home Cage (VEH and MET) and the MET – Stress groups.
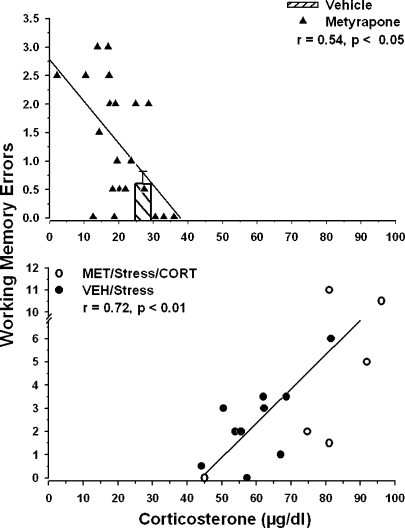
FIGURE 2.
Linear correlations between endogenous corticosterone levels and spatial memory errors. There was a significant negative correlation between corticosterone and spatial memory for animals in the VEH and MET-Home Cage groups and the MET-Stress groups (top). The hatched bar indicates the mean (± SEM) number of errors for the control (HC-No Stress) group plotted on the x axis at the mean corticosterone level for this group. The lower graph indicates that there was a significant positive correlation between corticosterone and errors for the VEH-Stress and METCORT/Stress groups (bottom).
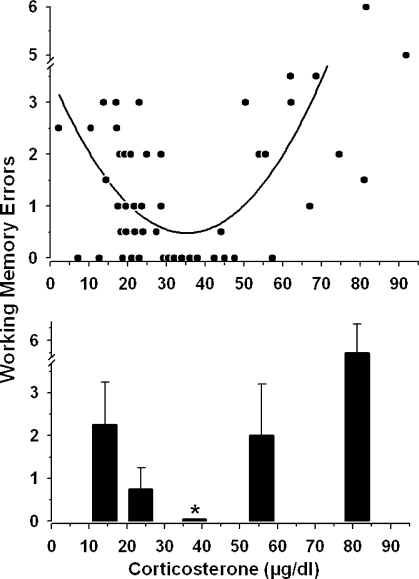
FIGURE 3.
Top: “U-shaped” line of best fit plotted through the data from Figure 2. To maximize resolution, this graph illustrates 96% (49/51) of the data points from Figure 2. The two data points offscale here (illustrated in Figure 2 at 96.1 and 80.0 ug/dl and 10.5 and 11 errors, respectively), were included in the line of best-fit analysis. Bottom: Data from all 51 subjects plotted as the median (± interquartile range) spatial memory errors for corticosterone levels from 0–18, 18.1–30, 30.1–50, 50.1–65 and 66–100. The x-axis values are plotted at the median corticosterone level for each group. The ∗ indicates that the middle group (30.1 – 50 ug/dl) was significantly different from the other 4 groups.
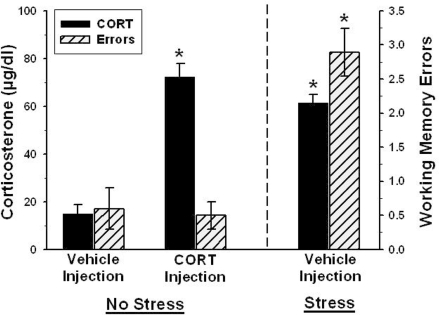
FIGURE 4.
There was no effect of corticosterone injection, alone, on spatial memory. Corticosterone levels are indicated on the left y-axis and spatial memory errors are indicated on the right y-axis. The two groups to the left of the vertical dashed line (Vehicle and corticosterone injection – Home Cage) were placed in their home cages during the 30 min delay and the group to the right of the dashed line was injected with the vehicle and placed near a cat during the delay. Corticosterone injection in rats placed in their home cages produced a significant increase in serum corticosterone levels, but did not increase memory errors. Cat-exposed rats had elevated endogenous corticosterone levels as well as increases in spatial memory errors, compared to the vehicle-injected home cage group. The ∗ indicates p < 0.05 compared to the home cage (control) group.
RESULTS
Baseline corticosterone levels
Baseline corticosterone levels were obtained from rats that were first given at least one week of daily water maze training. Then, a single blood sample was obtained from them at the time of day in which they would have been placed in the tank, i.e., about 1 hour after they were transported to the laboratory. The baseline corticosterone level was 2.5 ± 0.57 μg/dl (n=13), and is illustrated as the area of gray shading in Figure 1 (top) extending across the bars for the 5 experimental groups.
Post-Memory Testing Corticosterone Levels
Post-memory testing blood samples were obtained from rats in the experimental groups immediately after Trial 5 on the second day after the rats reached their DTC. Each of the 5 experimental groups had significantly higher corticosterone levels than the laboratory baseline group (F5,59 = 98.1, p < 0.001; SNK, p < 0.05), confirming that placement in the water was stressful.
The two hatched bars to the left of the vertical line in Figure 1 (top) indicate that training in the water maze, without cat exposure, produced an equivalent increase in corticosterone levels in the vehicle- and metyrapone-injected groups. Hence, metyrapone administration 30 min before Trial 1 did not block the increase in corticosterone levels produced by immersion in the water. The lack of metyrapone effect on corticosterone levels in this group was unexpected, but has been reported in another study (Kerr et al., 1994). In addition, the post-metyrapone corticosterone levels we observed here are comparable to those reported by Akirav et al., (2004), who also administered metyrapone prior to water maze training. It is possible that cold water exposure stimulated the release of corticosterone that had already been synthesized and stored in the adrenal glands (Cam and Bassett 1983) prior to the metyrapone injection.
The three filled bars to the right of the vertical line in Figure 1 (top) show the effects of the combination of maze training and cat stress on corticosterone levels in the Vehicle-injection (VEH), metyrapone injection (MET) and metyrapone + corticosterone injection (MET+CORT) groups. Immediately after the memory test trial, the VEH/Stress group had an increase in corticosterone levels which was significantly greater than corticosterone levels in the two home cage (VEH/No Stress and MET/No Stress; left side) groups and the MET/Stress group (SNK, p < 0.05). The group given metyrapone (30 min before Trial 1) followed by corticosterone injection and stress (MET+corticosterone/Stress) (during the delay period) had significantly greater levels of corticosterone than all of the other groups (SNK, p < 0.01).
Spatial Memory Performance
The graph in the bottom of Figure 1 illustrates Trial 5 (memory) performance. As with the top graph, the hatched bars to the left of the vertical line in the bottom of Figure 1 illustrate data for the two groups that spent the delay period in their home cages (control condition), and the filled bars on the right illustrate data for three groups that were exposed to the cat (stress conditions). Overall, there was a significant effect of group (F4,50 = 6.23, p < 0.001). There was no significant difference between the vehicle- and metyrapone-injected home cage groups (left side), indicating that metyrapone had no effect on memory under control conditions. The Vehicle-injected (VEH) and MET/CORT groups exposed to the cat (MET + CORT/Stress) committed significantly more errors than both groups of home cage-exposed rats and the metyrapone-injected stress group (MET/Stress) (SNK, p < 0.05).
Corticosterone-Memory Correlations
We performed additional analyses of the metyrapone-treated groups shown in Figure 1 and found that corticosterone levels of individual rats correlated with their spatial memory errors. Figure 2 (top) illustrates the finding that there was a significant negative correlation between corticosterone levels and errors for all rats given metyrapone (MET-HC and MET-Stress; r = 0.54, p < 0.05).
Rats trained under control conditions (VEH-HC) exhibited a narrow range of both corticosterone levels and memory errors. It was not feasible, therefore, to conduct a regression analysis between corticosterone and memory errors for this group. The corticosterone and memory data for the control group is provided as an overlay bar plot in the top of Figure 2. The mean number of errors and SEM for the control group overlaps with the regression line formed solely by the metyrapone-treated animals, suggesting that the corticosterone-memory correlation is continuous between the metyrapone-treated and control rats.
A regression analysis was also performed on the two groups with corticosterone levels that were increased by stress or by the combination of stress, metyrapone and corticosterone injection (VEH/Cat Exposure and MET + CORT/Stress). Figure 2 (bottom) shows that there was a significant positive correlation between corticosterone levels and memory errors for the rats in these two groups (r = 0.72, p < 0.01).
U-Shaped Relationship Between Corticosterone and Memory
Figure 3 is a comprehensive analysis of all of the raw data from the 5 experimental (non-baseline) groups (shown in Figures 1 and 2). The top graph is a line of best fit through the data (Sigmaplot 8.0, spline curve fit). The line is U-shaped, with the greatest incidence of errors occurring at the lowest and highest corticosterone levels and the fewest incidence of errors committed by animals with corticosterone from 30–50 μg/dl.
The lower graph in Figure 3 is a transform of the data to conduct a statistical analysis of the U-shaped function. The range of corticosterone was arbitrarily divided into five regions from 0–18 (n=10), 18.1–30 (n=18), 30.1–50 (n=9), 50.1–68 (n=7) and 68.1–100 (n=7) μg/dl, and the median values in each range (± the interquartile range) are shown. Non-parametric analyses were conducted because the data were not normally distributed. The fewest memory errors were committed by rats with corticosterone levels from 30.1–50 μg/dl (median errors = 0, interquartile range = 0), and significantly more errors were committed by rats with corticosterone levels outside of this mid-range (Kruskal-Wallis ANOVA on ranks, H=26.168, 4 df; post-hoc Dunn's Method, p < 0.05).
Effects of Exogenous Corticosterone on Memory
The basis of the stress-induced impairment of memory may have been the acute increase in corticosterone from optimal performance levels (30–50 μg/dl) to cat-stress evoked levels (> 50 μg/dl). To test this possibility, we injected a different group of rats with corticosterone (5 mg/kg, sc) at the start of the delay period and then they spent the delay period in their home cages. Thirty min later (immediately after the memory test trial) this group had an increase in serum corticosterone levels which was statistically equivalent to the increase in endogenous corticosterone levels found in vehicle-injected rats exposed to the cat (Figure 4). However, unlike cat-exposed rats, the corticosterone-injected home cage group did not commit a significant increase in errors (Figure 4). Therefore, increases in corticosterone correlated with impaired memory only when elevated levels occurred in cat-exposed rats.
DISCUSSION
Investigators have long suggested that the complex effects of emotionality on memory can be understood within the framework of a U-shaped dose-response function between GCs and hippocampal-dependent memory (Lupien and McEwen 1997; Cahill and McGaugh 1998; Conrad et al., 1999; Roozendaal, 2002; Lupien et al., 2005; Conrad, 2005). However, the effects of high levels of GCs or GC agonists on hippocampal-dependent memory, as well as LTP, can be blocked in animals with a suppression of functioning of the amygdala (Roozendaal et al., 1996; Roozendaal and McGaugh 1996; Kim et al., 2001; Roozendaal et al., 2003; Kim et al., 2005). It was therefore not known how there can be a dose-response “U-shaped” function between GC levels and hippocampal functioning which can be nullified by inactivation of the amygdala. Our findings have addressed this issue. We have shown that the expression of the corticosterone-memory dose-response function was dependent on the emotional context in which the elevated corticosterone levels occurred. High levels of corticosterone correlated with impaired memory only for rats that were placed in a fear-provoking, i.e., amygdala activating, condition. Thus, the group with endogenous increases in corticosterone produced by cat exposure and the group given metyrapone with corticosterone replacement, followed by cat exposure, both showed memory impairments. By contrast, the group with high levels of corticosterone produced by exogenous corticosterone administration without predator exposure exhibited intact memory. Our work, therefore, indicates that an elevated level of corticosterone, alone, does not impair spatial memory; elevated levels of corticosterone needed to occur in conjunction with the animal being in a fear-provoking environment for memory to be impaired.
Our finding that heightened emotionality is a critical factor in the manifestation of corticosterone effects on memory is consistent with the work of Buchanan and Lovallo (2001) in their study of cortisol-arousal interactions in people. These investigators reported that cortisol administration enhanced memory for emotionally arousing information in people, but had no effect on memory for emotionally neutral information. Brandenberger et al. (1980) also showed a positive correlation between stress-induced increases in cortisol levels and memory. Whereas in the Buchanan and Lovallo work, elevated levels of cortisol correlated with an enhancement of memory, Brandenberger et al showed that elevated levels of cortisol correlated with an impairment of memory. The capacity for GCs to either enhance or impair memory has been a topic of discussion in recent years. Investigators have noted that an increase in GC levels appears to contribute to the enhancement of the memory of the stress experience, itself, while simultaneously impairing retrieval of information acquired outside of the stress context (Sandi, 1998; Cordero et al., 1998; de Kloet et al., 1999; Diamond et al., 2001; Roozendaal, 2002). Thus, high GC levels at the time of learning have been shown to enhance the memory of an arousing experience in rats (Roozendaal and McGaugh 1997; Cordero et al., 1998) and people (Buchanan and Lovallo 2001), and to impair memory for information acquired outside of the stress experience (Diamond et al., 2004a; Diamond et al., 2004b). Our findings, therefore, are consistent with the literature on corticosterone-memory interactions in that the information that was forgotten (the platform location) was outside of stress context (cat exposure).
The lack of effect of exogenously administered corticosterone on spatial memory in rats that were not exposed to the cat described here replicates the findings of Sandi et al. (1997). These investigators found that corticosterone administration to rats which were trained under conditions similar to ours (water maze testing in cold water without cat exposure) did not impair spatial memory. Our findings are also relevant toward understanding why corticosterone levels can be elevated under a variety of different conditions, but memory is impaired in only a subset of those conditions. That is, stressful conditions, such as predator exposure in rats (Diamond et al., 1999; Woodson et al., 2003; Diamond et al., 2004a; Sandi et al., 2005) and public speaking in people (Kirschbaum et al., 1996; Wolf et al., 2001; Payne et al., 2002), are associated with increases in GC levels and with impaired memory. However, an increase in GC levels can also occur under other conditions, such as during feeding, exercise and sex, but these activities don't produce cognitive impairments (reviewed in Kim and Diamond 2002).
In work relevant to this issue, we have found previously that two different behavioral conditions each produced high corticosterone levels in rats, but spatial memory was impaired in only one of the two conditions. In that study, male adult rats were exposed to either a cat or a sexually receptive female rat. Cat and female rat exposure produced an equivalent elevation of endogenous corticosterone levels in the male rats, but only cat exposure produced a spatial memory impairment (Woodson et al., 2003). The differences between cat and female rat effects on spatial memory, in the absence of differences in corticosterone levels between the two manipulations, provides empirical support for the idea that the increase in corticosterone needs to occur in conjunction with a fear-induced behavioral state for memory to be impaired.
Our findings are also consistent with those from a study with similar methodological procedures. As with the current study, Akirav et al (2004) administered metyrapone to rats prior to the acquisition phase of training. These authors found that metyrapone administration to rats trained in cold water reduced corticosterone levels and impaired their performance in the acquisition and memory phases of testing. This group also did not report that corticosterone administration, alone, impaired learning and memory. On the contrary, in this work and in their previous study (Sandi et al., 1997), corticosterone given to rats trained under low stress training conditions actually enhanced spatial memory. Their findings, in conjunction with ours, provide strong support for the hypothesis that the effects of corticosterone on learning and memory are dependent on the context in which the elevation of the corticosterone occurs.
The basis of the permissive influence of stress in the expression of the correlation between elevated corticosterone levels and impaired spatial memory is not known. The process may involve the corticosterone-induced activation of intrinsic hippocampal GC (de Kloet et al., 1999) and NMDA (Kim et al., 1996) receptors, in conjunction with activation of the amygdala (Setlow et al., 2000; Kim et al., 2001; Kim et al., 2005). Specifically, the hippocampus contains the highest density of corticosterone receptors and administration of corticosterone agonists can affect hippocampal LTP (Rey et al., 1994; Pavlides et al., 1996; Alfarez et al., 2002) and spatial memory (Kim and Diamond 2002). However, under some conditions, elevated corticosterone levels have been shown to have no effect on LTP or memory. For example, acute administration of an antidepressant, tianeptine, blocked stress effects on LTP (Shakesby et al., 2002) without reducing the stress-induced rise of corticosterone levels. Similarly, Kim et al. (2001; 2005) showed that stress did not block LTP or impair memory in rats with damage to, or inactivation of, the amygdala, despite the fact that the stressed amygdala-lesioned rats had a normal stress-induced increase in corticosterone levels. Finally, Roozendaal and co-workers reported that corticosterone effects on hippocampus-dependent memory could be blocked by inactivation of the amygdala (Roozendaal, 2000; Roozendaal et al., 2003). These studies, in concert with the current findings, support the view that an increase in corticosterone levels is not a sufficient condition to mediate stress effects on hippocampal plasticity and learning. Thus, the stress-dependent correlation between elevated levels of corticosterone and memory that we observed here may have been produced by corticosterone actions directly on the hippocampus in conjunction with amygdala-hippocampus interactions (Richter-Levin and Akirav 2000; Diamond et al., 2001; Abe, 2001).
Although it is possible that activation of hippocampal GC receptors contributed to the stress effects on memory described here, the rapid time-course (in 30 minutes) argues against the effects occurring via classical steroid binding to intracellular receptors, followed by genomic activation and protein synthesis (de Kloet et al., 1998). It is more likely that rapid, i.e., non-genomic, actions of corticosterone influenced hippocampal functioning in the current work. It is known that peripheral administration of GCs can increase levels of excitatory amino acids in the hippocampus (Venero and Borrell 1999) and corticotropin releasing hormone (CRH) levels in the amygdala (Cook, 2002) in less than 30 min. Moreover, Cook (2002) reported that predator stress produced a rapid (within 2 min) increase in amygdala CRH levels, followed by a secondary increase of CRH levels 25 min after the onset of the stressor. By contrast, administration of a GC, to otherwise non-stressed animals, produced only the secondary (25 min) elevation of CRH levels. These findings indicate that the effects of elevated corticosterone levels in conjunction with predator exposure, as compared to elevated corticosterone levels produced by injection alone, can be distinguished on the basis of differences in production of amygdaloid CRH. Thus, interactions between the rapid stress-induced corticosterone-independent increase in CRH levels in conjunction with slower corticosterone-dependent increases in amygdaloid CRH (as well as stress-induced increases in amygdaloid norepinephrine (Roozendaal, 2000; McGaugh and Roozendaal 2002)) are likely to underlie the permissive influence of stress in the expression of corticosterone effects on spatial memory.
Whereas the high end of the U-function between corticosterone and memory impairments was produced by the combination of predator stress and elevated corticosterone levels, the low end of the U-function was produced by a reduction in corticosterone by metyrapone. Our finding of a correlation between impaired memory and reduced corticosterone levels is consistent with other work showing that a reduction in corticosterone levels by metyrapone (Loscertales et al., 1997; Liu et al., 1999; Cordero et al., 2002; Akirav et al., 2004), adrenalectomy (Oitzl and de Kloet 1992; Vaher et al., 1994; Conrad et al., 1997; Oitzl et al., 2001) or antagonism of corticosterone activity (Fleshner et al., 1997; Conrad et al., 1999; Oitzl et al., 2001) impaired hippocampus-dependent learning and memory, and that the impairment could be reversed by glucocorticoid replacement in rodents (Pugh et al., 1997) and people (Lupien et al., 2002a; Lupien et al., 2002b).
The observation of optimal memory performance by rats with intermediate corticosterone levels (30–50 μg/dl) deserves consideration. Water maze training is an inherently stressful task, as indicated by high corticosterone levels found in rodents subjected to the task (Diamond et al., 1996; Holscher, 1999) (Figure 1). Under control conditions, water maze-trained rats exhibited excellent spatial memory, despite the fact that the task evokes levels of corticosterone that are normally considered to be in the stress range. The elevated corticosterone levels produced by water maze training may, in fact, have produced positive effects on memory. This view of a facilitatory effect of intermediate levels of corticosterone on memory is supported by findings in which training conditions which typically produce low levels of corticosterone and impaired learning and memory resulted in significant improvements in performance with corticosterone supplementation (Sandi and Rose 1994; Sandi et al., 1995; Sandi et al., 1997; Cordero and Sandi 1998; Roozendaal et al., 1999). In related electrophysiological work, supplementation of adrenalectomized rats with corticosterone to produce an intermediate level of serum corticosterone resulted in an enhancement of the magnitude of primed burst (PB) potentiation (Diamond et al., 1992), a low threshold form of LTP (Rose and Dunwiddie 1986; Diamond et al., 1988). However, very high or low levels of corticosterone correlated with reduced PB potentiation (Bennett et al., 1991; Diamond et al., 1992). Similar findings from Kerr et al (1994) support the view that intermediate levels of corticosterone enhance processes involved in the storage of information.
In summary, we have found a U-shaped function between corticosterone levels and memory which is relevant to a large literature demonstrating that an acute increase or decrease in corticosterone levels can correlate with impaired memory. We also found that elevated corticosterone levels, alone, were insufficient to affect memory. The elevated corticosterone levels needed to occur in conjunction with a behavioral stress state for corticosterone-related memory impairments to be expressed. The stress-corticosterone interactions described here are potentially relevant toward understanding how amygdala activation interacts with stress levels of corticosterone to influence hippocampal processing.
ACKNOWLEDGMENTS
The authors thank Cheryl Conrad, Catherine Bennett and the reviewers for helpful commentary on earlier versions of this manuscript, and to Deric Macintosh for technical assistance. This research was supported by a VA Merit Review award to DMD.
Abbreviations
- LTP
long-term potentiation
- DTC
days to criterion
- corticosterone
corticosterone
- CRH
corticotropin releasing hormone
REFERENCES
- Abe K. Modulation of hippocampal long-term potentiation by the amygdala: a synaptic mechanism linking emotion and memory. Jpn J Pharmacol. 2001;86:18–22. doi: 10.1254/jjp.86.18. [DOI] [PubMed] [Google Scholar]
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learning & Memory. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarez DN, Wiegert O, Joels M, Krugers HJ. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience. 2002;115:1119–1126. doi: 10.1016/s0306-4522(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Bennett MC, Diamond DM, Fleshner M, Rose GM. Serum corticosterone level predicts the magnitude of hippocampal primed burst potentiation and depression in urethane-anesthetized rats. Psychobiol. 1991;19:301–307. [Google Scholar]
- Bower GH, Sivers H. Cognitive impact of traumatic events. Development And Psychopathology. 1998;10:625–653. doi: 10.1017/s0954579498001795. [DOI] [PubMed] [Google Scholar]
- Brandenberger G, Follenius M, Wittersheim G, Salame P. Plasma catecholamines and pituitary adrenal hormones related to mental task demand under quiet and noise conditions. Biol Psychol. 1980;10:239–252. doi: 10.1016/0301-0511(80)90037-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. Emotionality and the Yerkes-Dodson Law. J Exp Psychol. 1957;54:345–352. doi: 10.1037/h0049114. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinol. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. Release of corticosterone from the rat adrenal cortex in response to administration of (1–24)adrenocorticotrophin. J Endocrinol. 1983;98:173–182. doi: 10.1677/joe.0.0980173. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The nonlinear effects of acute glucocorticoid activity on hippocampal function. NonLinear Relationships in Biology, Toxicology and Medicine 2005. [DOI] [PMC free article] [PubMed]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiol LearningMemory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of Type I and Type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brn Res. 1997;759:76–83. doi: 10.1016/s0006-8993(97)00236-9. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 2002;75:455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behav Neurosci. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell A, Park CR, Vouimba RM. Preclinical research on stress, memory, and the brain in the development of pharmacotherapy for depression. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S491–S495. doi: 10.1016/j.euroneuro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behavioral Neuroscience. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposure to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Puls MJ, Rose GM. Differential effects of stress on hippocampal and amygdaloid LTP: Insight into the neurobiology of traumatic memories. In: Holscher C, editor. Neuronal Mechanisms of Memory Formation. New York: Cambridge University Press; 2001. pp. 379–403. [Google Scholar]
- Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Pugh CR, Tremblay D, Rudy JW. DHEA-S selectively impairs contextual-fear conditioning: support for the antiglucocorticoid hypothesis. Behav Neurosci. 1997;111:512–517. doi: 10.1037//0735-7044.111.3.512. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res. 2004;155:77–84. doi: 10.1016/j.bbr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Green B. Post-traumatic stress disorder: symptom profiles in men and women. Curr Med Res Opin. 2003;19:200–204. doi: 10.1185/030079903125001604. [DOI] [PubMed] [Google Scholar]
- Holscher C. Stress impairs performance in spatial water maze learning tasks. Behav Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Huggett AM, Abraham WC. Modulation of hippocampal long-term potentiation and long-term depression by corticosteroid receptor activation. Psychobiol. 1994;22:123–133. [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci U S A. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Res. 1999;821:134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- Loftus EF, Burns TE. Mental shock can produce retrograde amnesia. Memory & Cognition. 1982;10:318–323. doi: 10.3758/bf03202423. [DOI] [PubMed] [Google Scholar]
- Loscertales M, Rose SP, Sandi C. The corticosteroid synthesis inhibitors metyrapone and aminoglutethimide impair long-term memory for a passive avoidance task in day-old chicks. Brn Res. 1997;769:357–361. doi: 10.1016/s0006-8993(97)00735-x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Buss C, Schramek TE, Maheu F, Pruessner J. Hormetic influence of glucocorticoids on human memory. Non-Linear Relationships in Biology, Toxicology and Medicine 2005. [DOI] [PMC free article] [PubMed]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. [Review] [209 refs] Brain Research - Brain Research Reviews. 1997;24:127. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Kin NMKN, Meaney MJ, Nair NPV. Acute modulation of aged human memory by pharmacological manipulation of glucocorticoids. Journal of Clinical Endocrinology and Metabolism. 2002;87:3798–3807. doi: 10.1210/jcem.87.8.8760. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere A, Menard C, Kin NMKN, Nair NPV. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinol. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Psychogenic amnesia. Neuroimage. 2003;20(Suppl 1):S132–S138. doi: 10.1016/j.neuroimage.2003.09.010. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Nadel L, Jacobs WJ. Traumatic memory is special. Current Directions in Psychological Science. 1998;7:154–157. [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joels M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci U S A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Ogawa S, Kimura A, McEwen BS. Role of adrenal steroid mineralocorticoid and glucocorticoid receptors in long-term potentiation in the CA1 field of hippocampal slices. Brn Res. 1996;738:229–235. doi: 10.1016/s0006-8993(96)00776-7. [DOI] [PubMed] [Google Scholar]
- Payne JD, Nadel L, Allen JJ, Thomas KG, Jacobs WJ. The effects of experimentally induced stress on false recognition. Memory. 2002;10:1–6. doi: 10.1080/09658210143000119. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextualfear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Rey M, Carlier E, Talmi M, Soumireu-Mourat B. Corticosterone effects on long-term potentiation in mouse hippocampal slices. Neuroendocrinology. 1994;60:36–41. doi: 10.1159/000126717. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol. 2000;22:11–20. doi: 10.1385/MN:22:1-3:011. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinol. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol LearningMemory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, de Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc Natl Acad Sci U S A. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoidinduced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. European Journal of Neuroscience. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. European Journal of Neuroscience. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast. 1998;6:41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994;647:106–112. doi: 10.1016/0006-8993(94)91404-4. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP, Mileusnic R, Lancashire C. Corticosterone facilitates long-term memory formation via enhanced glycoprotein synthesis. Neuroscience. 1995;69:1087–1093. doi: 10.1016/0306-4522(95)00306-4. [DOI] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry. 2005;57:856–864. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci. 2000;12:367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett RG. The relationship of performance level to level of arousal. J Exp Psychol. 1957;54:54–61. doi: 10.1037/h0043340. [DOI] [PubMed] [Google Scholar]
- Vaher PR, Luine VN, Gould E, McEwen BS. Effects of adrenalectomy on spatial memory performance and dentate gyrus morphology. Brain Res. 1994;656:71–78. doi: 10.1016/0006-8993(94)91367-6. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Watters PA, Martin F, Schreter Z. Caffeine and cognitive performance: The nonlinear Yerkes-Dodson Law. Human Psychopharmacology-Clinical and Experimental. 1997;12:249–257. [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinol. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- Yovell Y, Bannett Y, Shalev AY. Amnesia for traumatic events among recent survivors: a pilot study. CNS Spectr. 2003;8:676–5. doi: 10.1017/s1092852900008865. [DOI] [PubMed] [Google Scholar]