Abstract
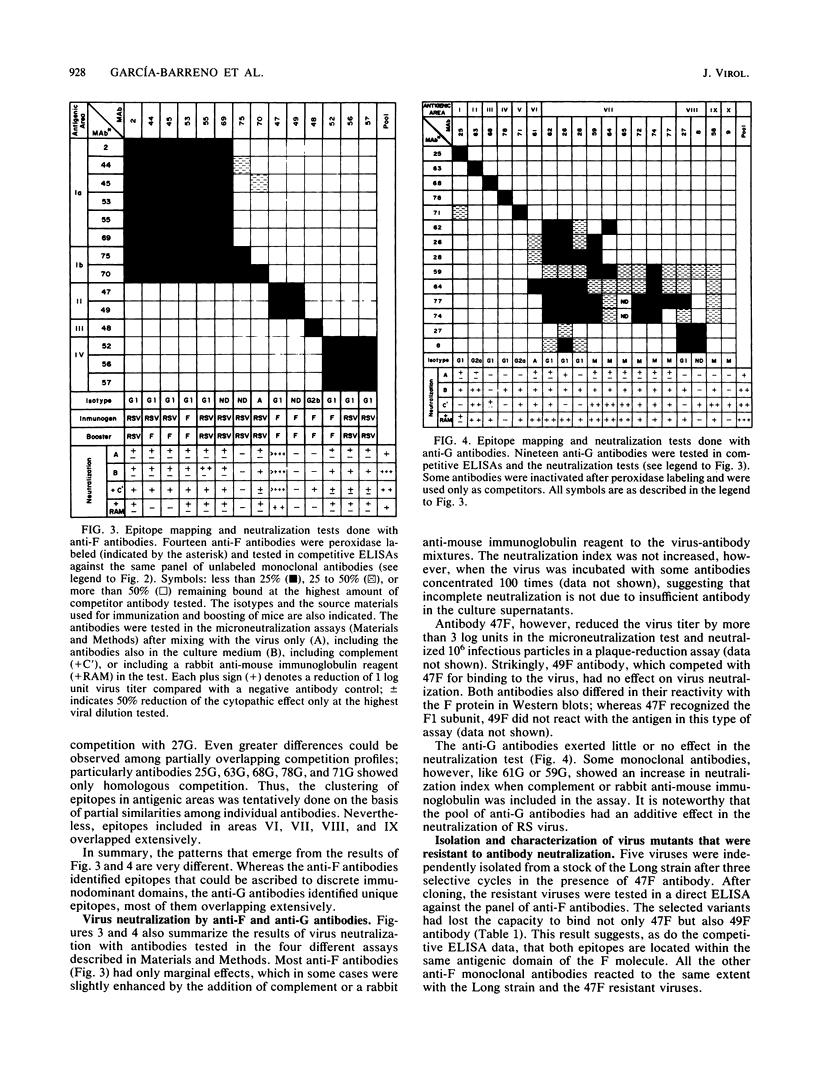
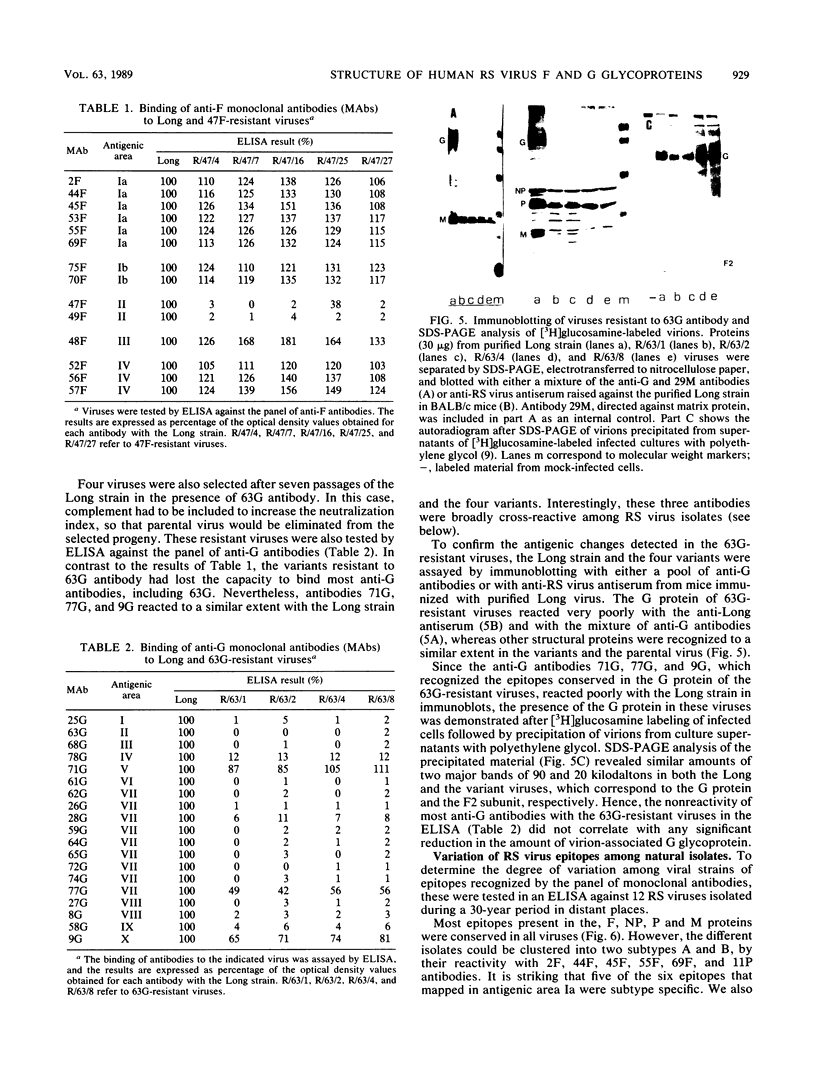
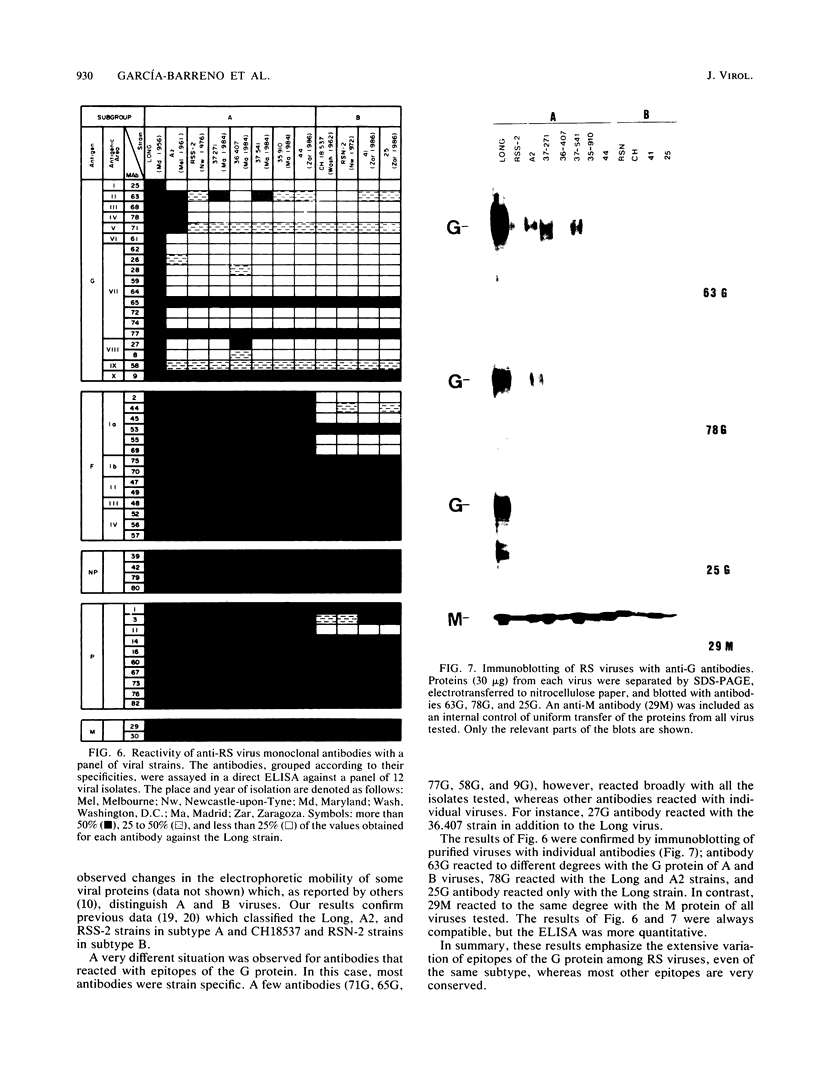
Monoclonal antibodies directed against the glycoproteins of human respiratory syncytial virus were used in competitive enzyme-linked immunosorbent assays for topological mapping of epitopes. Whereas epitopes of the F glycoprotein could be ascribed to five nonoverlapping antigenic sites, anti-G antibodies recognized unique epitopes, many of whose competition profiles overlapped extensively. Variant viruses selected with a neutralizing (47F) anti-F antibody lost the binding for only 47F and 49F antibodies, which mapped in the same antigenic area. In contrast, viruses selected with an anti-G antibody lost the capacity to bind most of the anti-G antibodies, and their G protein was not recognized by an anti-virus antiserum, indicating major changes in the antigenic structure of the G molecule. Finally, we found great antigenic variation of the G protein among viral isolates. This occurred even within viruses of the same subtype with only limited divergence of amino acid sequence between strains. All of these data indicate marked differences in the antigenic organization of the G and F glycoproteins of respiratory syncytial virus; we discuss these differences in terms of the chemical structure of the glycoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlind B., Norrby E. Occurrence of respiratory syncytial virus subtypes A and B strains in Sweden. J Med Virol. 1986 Jul;19(3):241–247. doi: 10.1002/jmv.1890190306. [DOI] [PubMed] [Google Scholar]
- Alexander S., Elder J. H. Carbohydrate dramatically influences immune reactivity of antisera to viral glycoprotein antigens. Science. 1984 Dec 14;226(4680):1328–1330. doi: 10.1126/science.6505693. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt H. N., Pringle C. R. Molecular cloning and sequencing of the F and 22K membrane protein genes of the RSS-2 strain of respiratory syncytial virus. J Gen Virol. 1987 Nov;68(Pt 11):2789–2796. doi: 10.1099/0022-1317-68-11-2789. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Coates H. V., Alling D. W., Chanock R. M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- Garcia-Barreno B., Jorcano J. L., Aukenbauer T., López-Galíndez C., Melero J. A. Participation of cytoskeletal intermediate filaments in the infectious cycle of human respiratory syncytial virus (RSV). Virus Res. 1988 Mar;9(4):307–321. doi: 10.1016/0168-1702(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Gimenez H. B., Hardman N., Keir H. M., Cash P. Antigenic variation between human respiratory syncytial virus isolates. J Gen Virol. 1986 May;67(Pt 5):863–870. doi: 10.1099/0022-1317-67-5-863. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983 Apr;64(Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R. Nucleotide sequence of the respiratory syncytial virus phosphoprotein gene. J Gen Virol. 1985 Jul;66(Pt 7):1607–1612. doi: 10.1099/0022-1317-66-7-1607. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Guillen M., Sanchez-Fauquier A., Melero J. A. An antigen-binding assay to determine the specificity of monoclonal antibodies against influenza virus and mapping of epitopes. J Virol Methods. 1986 Jun;13(3):255–264. doi: 10.1016/0166-0934(86)90019-4. [DOI] [PubMed] [Google Scholar]
- López J. A., Villanueva N., Melero J. A., Portela A. Nucleotide sequence of the fusion and phosphoprotein genes of human respiratory syncytial (RS) virus Long strain: evidence of subtype genetic heterogeneity. Virus Res. 1988 May;10(2-3):249–261. doi: 10.1016/0168-1702(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Morgan L. A., Routledge E. G., Willcocks M. M., Samson A. C., Scott R., Toms G. L. Strain variation of respiratory syncytial virus. J Gen Virol. 1987 Nov;68(Pt 11):2781–2788. doi: 10.1099/0022-1317-68-11-2781. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E., Mufson M. A. Preparation and characterization of monoclonal antibodies directed against five structural components of human respiratory syncytial virus subgroup B. J Gen Virol. 1987 Dec;68(Pt 12):3125–3135. doi: 10.1099/0022-1317-68-12-3125. [DOI] [PubMed] [Google Scholar]
- Satake M., Coligan J. E., Elango N., Norrby E., Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985 Nov 11;13(21):7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fauquier A., Villanueva N., Melero J. A. Isolation of cross-reactive, subtype-specific monoclonal antibodies against influenza virus HA1 and HA2 hemagglutinin subunits. Arch Virol. 1987;97(3-4):251–265. doi: 10.1007/BF01314425. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E. E., Brandriss M. W., Schlesinger J. J. Purification and characterization of the respiratory syncytial virus fusion protein. J Gen Virol. 1985 Mar;66(Pt 3):409–415. doi: 10.1099/0022-1317-66-3-409. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]