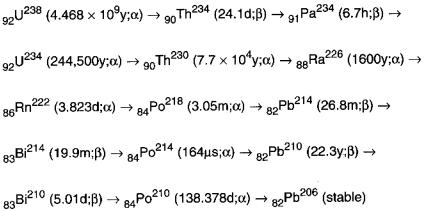Abstract
In spite of long traditions, treatments utilizing radon-rich air or water have not been unequivocally embraced by modern medicine. The objective of this work is to examine factors that contribute to this continuing controversy. While the exact mechanism of radon's effect on human body is not completely understood, recent advances in radiobiology offer new insights into biochemical processes occurring at low-level exposures to ionizing radiation. Medical evidence and patients' testimonials regarding effectiveness of radon spa treatments of various ailments, most notably rheumatoid arthritis are accumulating worldwide. They challenge the premise of the Linear-No-Threshold (LNT) theory that the dose-effect response is the same per unit dose regardless of the total dose. Historically, such inference overshadowed scientific inquiries into the low-dose region and lead to a popular belief that no amount of radiation can be good. Fortunately, the LNT theory, which lacks any scientific basis, did not remain unchallenged. As the reviewed literature suggests, a paradigm shift, reflected in the consideration of hormetic effects at low-doses, is gaining momentum in the scientific community worldwide. The impetus comes from significant evidence of adaptive and stimulatory effects of low-levels of radiation on human immune system.
Keywords: radon, radiobiology, hormesis
INTRODUCTION
Archeological discoveries in Central and Southern Europe suggest that therapeutic properties of certain waters were well recognized in the ancient times, but only within the last century high concentrations of radon were discovered to be present in these waters. Natural and artificial grottoes, and carved in the rock bathing tubs, found in some of the oldest spas in this region such as the island of Ischia in the volcanic area near Naples, Merano [2,000 Bq/L] and Lurisia [4,000 Bq/L] spas in the alpine region, and others (Pratzel HG and Deetjen P 1997), point to the use of treatments involving the intake of radon gas through inhalation, or by transcutaneous resorption of radon dissolved in water.
HISTORICAL ACCOUNTS PRIOR TO THE DISCOVERY OF RADON
Ancient Greeks enjoyed baths in natural waters for cleansing and hygienic purposes and took the waters after extensive exercise. With time, additional benefits were ascribed to bathing. Hippocrates [460-370 BC] put forth a hypothesis that an imbalance of the bodily fluids was at the core of all diseases and proposed that a change in the human environment and lifestyles would help; specific recommended activities included bathing, perspiration, walking and massages. The Romans, influenced by these traditions, developed their own multipurpose spa systems. Thermal baths at mineral and thermal springs were used not only for rest and recreation, but also served as medical facilities. Around 124 BC, a Greek physician Asclepiades who practiced in Rome introduced general hydrotherapy for both therapeutic and preventive purposes, and drinking cures. Hydrotherapy included immersion of the whole body in water in order to treat rheumatic and urogenital disorders, as well as application of water to afflicted parts of the body (Jackson RPJ 1999).
Interesting possibilities of derivation of the term “spa” are explored in a recent article published in Annals of the Rheumatic Diseases (Van Tubergen A and van der Linden S 2002). Among the most plausible is that “spa” is the acronym of the Latin phrase “sanitas per aquas” [health through water]. In various literature sources, bathing in thermal waters for therapeutic purposes is described by different terms such as balneotherapy, spa therapy, hydrotherapy, taking the waters, and others. Throughout the centuries the interest in the use of water in medicine has fluctuated from nation to nation and the opinions about these uses ranged from considering them beneficial to harmful.
Preceding the discovery of what now is known as radon, and its reported benefits, there have been numerous observations of detrimental health among miners in Central Europe. First warnings about “dangerous air in the depth of the earth” were documented in the 15th century, and about 1530, the famous Swiss-German physician Paracelsus, or more properly Theophrastus Phillippus Aureolus Bombastus von Hohenheim [1493–1541], who practiced in mining towns of the region, brought “Bergsucht” or “mountain disease” to wider attention. Reports by other contemporaries followed. Only after the discovery of ionizing radiation and “emanation”, radon became a suspect (Becker 2003).
RADON DISCOVERY AND ITS SIGNIFICANT PROPERTIES
The end of the 19th century was marked by major scientific discoveries, of X-rays by a Dutch-German physicist K.W. Roentgen [1845–1923] and radioactivity by a French scientist A.H. Becquerel [1852–1908] among them. The discovery of radium [226Ra] by a Polish lady scientist M. Sklodowska-Curie and her husband, French professor P. Curie followed. Only two years later, in 1900, a daughter product of radium, radon [Rn-222] was discovered by a German chemist F.E. Dorn who called it “radium emanation”. Radon also initially called “niton”, was separated by E. Rutherford [1871–1937] and F. Soddy [1877–1956] in 1902 (Draganic IG et. al. 1990).
Radon is an inert gas with atomic number of 86; the mass of the most stable isotope is 222. It naturally occurs as a result of α decay of 226Ra, which in turn is derived from the long-lived antecedent, uranium (238U) as depicted in Figure 1.
FIGURE 1.
U-238 naturals decay series. Subscripts refer to atomic numbers and superscripts refer to mass numbers. Half-lives and α or β types of emission are shown in parentheses. All members of the series also emit one or more γ except Bi-210 and stable Pb-206. All β emissions are β−1 (after Hallenbeck 1994).
Of the three naturally occurring radon isotopes, 219Rn [actinon, half-life under 4 seconds, occurring in the 235U decay series], 220Rn [toron, half-life of just under 1 minute, occurring in the 232Th decay series], 222Rn [radon, half-life 3.825 days], the latter, with biological half-life of about 30 minutes, is biologically most significant.
This colorless, odorless, and chemically inactive gas is 7.6 times heavier than air; it readily dissolves in water, particularly if water is slightly acidic and not rich in minerals, and in alcohol and fatty acids. Alpha radiation with 5.49 MeV energy emitted by radon operates on short distance with penetration ability of about 41.1 μm in water and about 20 μm in tissue (Deetjen 1990).
Among radon daughters, 218Po, 214Pb, 214Bi, and 214Po, traditionally referred to as radium A, radium B, radium C and radium C', respectively, are most important from the standpoint of balneology as they comprise a radioactive sediment. Residence time of radon in human organism is short—it is estimated that about 59% of radon is eliminated within 15–30 minutes. The final decay of radon in the organism to the level not detectable by analytic methods takes place within 2–3 hours. After that time, only the sediment remains in the system; it emits mostly α and β radiation, and photons only to a minor extent, during the first 7 hours of residence in the system, resulting in the creation of radium D+E [210Pb and 210Bi], which becomes a long-term [half-life of 22.3 years] source of weak β radiation (Deetjen and Jockel 1992, Hallenbeck 1994).
RECENT UNSETTLING EVENTS
While interest in the bathing culture grew throughout the world during the 19th and 20th centuries, the opinions about radon treatments ranged from very enthusiastic to extremely critical. A number of factors influenced public opinion.
During the 20th century, high radon concentrations have been observed in uranium and non-uranium mines, and at uranium milling sites in Australia [Radium Hill and other uranium mining sites], Canada, China, Czechoslovakia [now Czech Republic and Slovakia], France, Germany, Sweden, the U.S. and other countries. It has been theorized that excess lung cancers among the underground miners results from exposure to radon and its daughters, 218Po and 214Po in particular. While there is no doubt that exposures to high radon concentrations contribute to the increase in lung cancer rates in this population, the degree of this contribution remains an unresolved issue due to the presence of smoking (Hallenbeck 1994), exposure to crystalline silica (Bochman 2001), diesel exhaust (USEPA 2002) and perhaps other carcinogens (Duport 2002). It is generally accepted among experts that there is no increased lung cancer risk at 500–1000 WLM [Working Level Month corresponds to exposure over 170 hours at 1 WL, where Working Level is equal approximately to 7.4 kBq/m3 or 200 pCi/L] (Becker 2003).
Discoveries of elevated indoor radon levels in Germany in the late 1940s, followed by similar findings in Sweden (Becker 2003) and in the U.S. [Grand Junction, CO] in the 1950s (Cole 1993), triggered a surge of scientific and public interest in the residential radon issue. Numerous surveys and epidemiological studies were initiated in various countries (Marcinowski 1992). Their findings led to meta-analyses (i.e. Lubin LH and Boice JD 1997) that followed a similar approach to that used by in earlier meta-analyses of underground miner studies. In spite of pitfalls inherent in meta-analyses, inadequate consideration of confounding factors and dosimetric inadequacies repeatedly pointed out by B. Cohen over the past ten years (most recently: Cohen B 2005), arbitrary linear extension of dose-response curve from high dose region to the origin of coordinates utilized in these analyses, became the basis of official risk models.
The most recent article that used the data pooled from several case-control studies (Krewski et al. 2005) is cited on the USEPA website link (see reference) as providing “direct evidence of an association between residential radon exposure and lung cancer below EPA's action level of 4 pCi/L [148 Bq/m3]”. It is further stated in a circulatory manner that “these results are consistent with radon risk estimates from the EPA's Assessment of Risk from Radon at Homes (EPA 402-R-03-003, June 2003), and the Health Effects of Exposure to Radon: BEIR VI, National Research Council, NAS 1999”.
Lung cancer is the leading cause of cancer deaths in American men and women (ACS website, CDC-National Vital Statistics Report 2003). A number of factors are associated with differences in the rates, including age, gender, racial/ethnic origin, and geographic location. According to a published by the EPA map, Colorado is one of the states with high residential radon concentrations. Indeed, only 12 out of 62 counties have radon concentration levels below 4pCi/L [148 Bq/m3] thus one would anticipate higher rates of lung cancer in the state.
On contrary, the overall incidence rate in Colorado was 54.7 versus 67.7 nationwide, per 100,000, in 2001. The 1999–2001 data available for the counties indicate that the average annual rate for the state was 53.1 per 100,000 with only 5 counties exceeding the U.S. incidence rate. Three of these counties were sites of extensive uranium mining and/or milling activities: Fremont [Cotter Mill], Mesa [Climax Uranium Mill], and Moffat [Maybell Mill]. It seems likely that some of the excess of lung cancer in these counties can be attributed to occupational rather than residential radon exposures.
The annual death rate due to lung and bronchus cancer in Colorado [41.8 per 100,000 in the 1998–2002 period] met the Healthy People 2010 objective No. 03–02 [44.9] (CDC), while the national death rate was considerably higher [55.7 per 100,000]. None of the Colorado counties exceeded the annual U.S. death rate during this period.
The Colorado example suggests that EPA's assumption of radon risk estimates at low levels is speculative. Similar observations regarding inconsistencies in alleged radon health threat have been made in other parts of the world, most notably in Japan and Iran. Japanese doctors report that people who live in the Misasa villages, where drinking water contains high concentration of radon and indoor radon level is about 54 Bq/m3 [1.46 pCi/L], about 3 times higher than the national mean (Yamaoka et al. 2005), exhibit far lower rates of cancer than elsewhere in Japan (Mifune et al. 1992), with cancer death rates of 3.66% versus 6.68%. Ramsar, Iran is another example. The region has some of the highest recorded levels of natural background radiation [i.e. 25 times higher than the average value for the U.S.], yet there is no increase incidence of leukemia or other cancers in this region (Ghiassi-nejad et al. 2002). Unreasonable assumption of radiation risks at low exposure levels adds to public's confusion and mistrust.
CURRENT STATUS OF RADON THERAPY
Radioactive radon gas is widely considered to be health hazard by environmental agencies in the U.S. and Europe (USEPA 1993, Becker 2003). Yet, despite the warnings of these agencies, thousands of people annually expose themselves to radon for therapeutic purposes in facilities ranging from rustic old mines to upscale spas and clinics (Erickson 2004).
It is important to realize that the renewed interest in radon therapy in recent decades has little relation to the popular fashion in the early part of 20th century, mostly in Europe that attributed a multitude of healing powers to radium. In addition to the use of external sources such as radium-impregnated bed blankets and compresses, Ra intake was practiced as well. Radium was, for example, added to many “health food” items and toothpaste (Becker 2004). While some of these uses may be viewed from the perspective of time as irresponsible, low doses of radiation, though not well controlled, may have been in some way beneficial to people of that era.
Well-known curative spas that operated in the last two centuries remain as historical monuments throughout Europe and other parts of the world. In many countries in Europe (Germany, Austria, France, Italy, Hungary, Bulgaria, Poland, the Czech Republic and others), South America (Chile, Brazil), and Asia (China, Japan) new centers have been constructed and their operation is frequently harmonized with modern medical approaches to achieve curative effects.
Among the traditional spas with high radon concentration, Bad Gastein, Austria is perhaps among the best known. Radon concentration in the air at the Gastein Healing Gallery averages 43 kBq/m3 [about 1.2 nCi/L] with maximal value of 160 kBq/m3 [about 4.3 nCi/L]. The breathing air at the gallery has temperature around 40°C and may be saturated with humidity if indicated. A classical cure at the Gallery provides a guest with a radiation dose of about 2.3 mSv (230 mrem) per year.
The immersion of the body in thermal water for therapeutic purposes is also practiced at Bad Gastein. Bathing water temperature markedly exceeds the recommended minimum of 20°C and the concentration on radon is in the range of 20 nCi/L [740 Bq/L] (Deetjen 1997).
In numerous descriptions and web advertisements of therapeutic spas in Europe, concentration of radon in thermal waters is expressed in the traditional units of Mache, named after German scientist H. Mache (1876–1954). The Mache unit [ME] is defined as “that quantity of Rn per liter, which without decay products and with complete utilization of the α-particles can maintain by its ionization of air a saturation current of 10 e.s.u…” (Curie et al. 1931; p 232). One ME corresponds to 13.5 Bq/L or 364 pCi/L. Thus concentration of radon in therapeutic waters used in Bad Gastein would be in the range 55 ME.
Historically, high values of radon concentration have been claimed by operators of various spas to remain competitive. However, claimed radon concentrations in the air of caves, former mine shafts, and other underground facilities require careful consideration of the conditions and duration of the measurements, as relatively large seasonal fluctuations are possible (Szerbin 1996).
While the concerns of environmental agencies in Europe about the risks of indoor radon levels are similar to those in the U.S., these concerns do not seem to preclude medical uses of radon. Most European countries tend to incorporate within their biomedical healthcare systems many of the genres of treatment categorized in the U.S. as “alternative” or “complementary” (i.e. homeopathy, manipulative techniques, naturopathy); in many countries such therapies are a covered medical benefit. Radon therapy is an established treatment that builds on centuries-old spa therapies (Erickson 2004). Many specialists, especially dermatologists and rheumatologists, now acknowledge the medical significance of bathing particularly when combined with other treatments such as physical exercise (Routh et al. 1996).
In addition to previously mentioned Bad Gastein in Austria, the currently best-known radon treatment centers with medical supervision are: Bad Hofgastein in Austria; Plombieres in France; Bad Kreunznach, Bad Munster and Schlema in Germany; Ischia in Italy; Misasa in Japan; and Pyatigorsk in Russia (Becker 2004). There are numerous other less known radon therapy centers such as Ikaria in Greece, Jachymov in the Czech Republic, Jahuel Hot Springs in Chile, Hisarja in Bulgaria, Felix Spa in Romania and many others.
Many health conditions are treated at these centers, with some success (Bad Gastein website link), inflammatory rheumatic illnesses such as Morbus Berchterew (ankylosing spondylitis), chronic polyarthritis, fibromyalgia, scleroderma, rheumatoid arthritis, and degenerative and deforming joint infections [arthrosis, spondylosis, osteochondrosis] among them. Other types of ailments include neuralgia, chronic pain such as one experienced as a result of a trauma, as well as respiratory diseases [bronchial asthma, chronic bronchitis, sinusitis], and allergic illnesses such as hay fever and neurodermitis. Treatments of complications involving the endocrine system, menopausal symptoms, impotence, and many other conditions are attempted as well.
There are about 40 health resorts or spa towns in Poland that provide the full range of services. Each facility has a specific profile, natural resources, a medical balneology department, professional medical staff, and a unique therapeutic climate for a range of illnesses. Not all engage in radon treatments. To be classified as a radon mineral water, it must have a minimum of 2 nCi/L (74 Bq/L) concentration of radon. Radon baths are typically used for hypertension, rheumatoid arthritis and arteriosclerosis of lower extremities, and inhalation therapy is often administered at speleotherapy centers for conditions such as chronic bronchitis and bronchial asthma. Compared to baths, inhalation radon is absorbed at a faster rate via mucous membrane (Ponikowska et al. 2002).
Among health resorts in the Lower Silesia in the former German lands in Poland, Szczawno Zdroj [Bad Salzbrunn], Ladek Zdroj [Bad Landeck], Swieradow (Bad Flinsberg) are among the centers where substantial clinical research is carried out. The latter two resorts, due to high radon content in water, are particularly attractive to patients with peripheral blood vessel disease (Joss et al. 2002). Department and Clinic of Endocrinology and Diabetes of the School of Medicine in Wroclaw [Breslau], Poland is actively involved in clinical studies of radon in the treatment of endocrine system disorders (Zdrojewicz and Belowska-Bien 2004a). Some of our recent findings are included below with those of others.
DO RADON TREATMENTS WORK AND HOW?
Until recently, other than causing lung cancer, the effects of human exposure to radon received little attention in the scientific literature. Reports issued more than a decade ago by the United Nations Scientific Committee on the Effect of Atomic Radiation (UNSCEAR 1993 and 1994) suggested adaptive responses to radiation by cells and organisms. Results of subsequent clinical trials and molecular-level studies as well as patients' testimonials point to beneficial rather than detrimental responses at low-level exposures. No fatalities have been observed due to participation in radon therapy.
Waters with radon appear to have analgesic, anti-inflammatory properties and provide neuro-vegetative balance. Among the conditions that have been treated in radioactive spas with greatest success, several, including rheumatoid arthritis, bronchial asthma, psoriasis, are described as having autoimmune etiology. They are characterized by an excessive response of the immune system, which then attacks individual's own cells (Soto 1997).
In a recent randomized sham-control study of a long-term efficacy of radon spa therapy in rheumatoid (Franke et al. 2000), subjects participated in a 4-week program in Bad Brambach, Germany consisting of complex regimen that included physical therapy. Cases participated in baths in natural spring water containing on average 1.3 kBq/L. Both groups showed marked effects at discharge in terms of reduction in pain intensity, the effects in the control group were short-lasting while cases enjoyed small to moderate long-lasting effects.
Studies that combine radon therapy with exercise are of particular interest for treatment of ankylosing spondylitis as they clearly indicate the superiority of this approach (Van Tubergen et al. 2001, Van Tubergen and Hidding 2002).
Radon therapy appears to aid in the recovery of the immune system. This is the case with bronchial asthma and even more so in the treatment of atopic asthma (Marshalick and Fenco 1991), as well as with intestinal dysbactreriosis (Marshalick and Shkolenko 1993), where long-lasting effect on the immune system has been demonstrated.
A series of on-going clinical studies involving radon therapy are conducted at the School of Medicine in Wroclaw, Poland. Their purpose is to investigate the degree of stimulating effect of this approach on the secretion of hormones. In these case-control studies, the regimen for both groups includes gymnastics and other spa activities. Case subjects undergo additional cyclical 15-minute immersions in radon water at the temperature of 37°C. These radon therapies are generally performed in Swieradow Zdroj, where bathing water with about 707 Bq/L [19 nCi/L] radon concentration is used. The duration of therapy varies with different health conditions.
In a study involving 54 menopausal women treated for trauma-related motion disorders, radon group showed statistically significant increase in the concentration of blood estradiol during therapy and only a slight decrease upon its completion (unpublished work in-progress). Similar stimulatory effects were observed in a study of 79 men [average age: 48]—a statistically significant increase in testosterone levels was observed in both controls and cases, though it was more pronounced in the radon group during therapy. Again, there was a decrease in blood concentration of testosterone in the radon group upon completion of therapy (Zdrojewicz and Bielowska-Bien 2004b). These decreases in the level of estradiol and testosterone upon removal of the stimulatory agent may be indicative of an adaptive process. The importance of follow-up studies in this are cannot be overstated. They may provide information on optimal treatment regimen with maximum duration of benefits. In effort to further investigate the effectiveness of radon therapy on the endocrine system, prolactin levels were assessed in both men and women, however no changes in the secretion of this hormone were observed (unpublished work in-progress). Additional inquiries in this area may provide explanation and perhaps better treatment of many immunological disorders.
The exact mechanism of radon's effect on human body is not completely understood. However, the most favored hypothesis is that radon's action is mediated by the neuroendocrine system, stimulation of the suprarenal glands by the hypophysis, rather than by direct action on the T cells. Irradiation would act on neurosecretory cells and lead to hormonal changes, which would then act on the different T cell subsets (Soto 1997, Liu et al. 1993).
The operation of small doses of radiation is complex. On the one hand, radiation may increase production of free radicals, on the other, it activates protective mechanisms responsible for their neutralization and it prevents expression of damaging actions of radicals. This mechanism is the basis of beneficial effects of small doses of ionizing radiation. All transformations of free radicals are associated with the formation of reactive oxygen species [ROS] (Halliwell 1994). Free radicals may influence the transmission of intercellular signals and impact biochemical processes such as proliferation and apoptosis (Jajte 1997). The main mechanisms protecting against damaging factors in cells are those that prevent the formation of ROS, mechanisms that eliminate earlier formed ROS (catching and extinguishing), and mechanisms that remove damaged molecules from the system to prevent their accumulation (Bergendi et al. 1999).
Both enzymatic and non-enzymatic protective mechanisms are available to cells. Among the enzymes, superoxide dismutase (SOD) deserves particular attention. SOD and p53 gene are considered stress response proteins. It has been shown that routine exposure to radon induces traces of active oxygen [in vivo in Misasa, Japan residents]. Elevated p53 levels indicate potentiating cancer-suppressive gene protein thus apoptosis may readily occur. While SOD activity generally decreases with age, Misasa female residents' SOD activity exceeded the reference range despite advanced age indicating increased antioxidant function (Yamaoka 2005). These factors may explain low cancer-related mortality in this region.
As mentioned earlier, while concerns of environmental agencies about indoor radon in many parts of the world are similar to those in the U.S., controlled radon therapy is pursued in a completely different cultural context. In radon curative tunnels such as the Gastein Healing Gallery in Austria, where radon is inhaled, patients are examined and a specific dose of radon prescribed for their individual conditions (Erickson 2004). In the words of Dr. Hornatova, former medical director of the Radium Palace in the Czech Republic, radon treatment is not considered “alternative therapy” nor is it considered to be a “nature cure”. Rather, it is simply one of many science-based treatment possibilities (Franke 2000).
No medical personnel of any kind are available at the Montana mines where radon treatment is passive and self-directed. At the better-known Free Enterprise Radon Health Mine, concentration of radon in the mine is tested on a regular basis by independent laboratories (Erickson 2004), with results ranging from 1.1 to 1.6 nCi/L [41–59 kBq/m3]. This represents 25–37% of the radon concentration at the Gastein Healing Gallery. According to Erickson (2004), people who use radon therapy in the U.S. are predominantly elderly, many with chronic illnesses, many of them report that medications they have been taking lost their effectiveness over time.
CONCLUSIONS
Central to radiation risk estimates based on the LNT hypothesis is the assumption that the dose-effect response is the same per unit dose regardless of the total dose. This suggests that the mechanisms of actions that induce these responses at low doses and at high doses are the same. This simply is not the case. Results of new cellular and molecular research indicate that the responses differ at different dose levels and it is possible to detect corresponding changes in gene regulation. One set responds to low doses, and another set of genes to high doses; these sets are both up- and down- regulated by radiation exposure (Aurengo 2005).
In light of recent clinical observations and the newest reports, it is clear that beneficial action of low doses of radiation clearly outbalances its potential risk. Hormesis, which is becoming a central issue in toxicology (Calabrese and Baldwin 2003), needs to be strongly considered in the investigations of low-dose radiation effects in humans. Radon therapy should continue to be studied with an open mind and scientific evidence for its efficacy diligently collected. Ascribed to Paracelsus phrase “It is the dose which makes the poison” remains relevant more than ever.
ACKNOWLEDGMENT
The authors would like to acknowledge clinical contribution by Dr. Kinga Belowska-Bien at the Department and Clinic of Endocrinology and Diabetes, School of Medicine in Wroclaw, Poland, and the late Dr. John Cameron for his seemingly tireless and tenacious efforts in investigating the hormetic zone of ionizing radiation.
REFERENCES
- ACS (American Cancer Society) website. Available at: http://www.cancer.org
- Aurengo A, Averbeck D, Bonnin A et al. [Tubiana M, Chairman]. 2005. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionizing radiation. Academie des Sciences [French Academy of Sciences]-Academie nationale de Medecine [National Academy of Medicine]. Report issued March 2005. Available at: http://www.academie-sciences.fr/publications/rapports/pdf/dose_effet_07_04_05_gb.pdf Bad Gastein website link available at: http://www.gastein.com
- Becker K. Health Effects of High Radon Environments in Central Europe: Another Test for the LNT Hypothesis? Nonlinearity in Biology, Toxicology and Medicine. 2003;1:3–35. doi: 10.1080/15401420390844447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K. One century of radon therapy. Int. J Low Radiation. 2004;1(3):334–357. [Google Scholar]
- Bergendi L, Benes L, Durackova Z, et al. Chemistry, physiology and pathology of free radicals. Life Sci. 1999;146:369–373. doi: 10.1016/s0024-3205(99)00439-7. [DOI] [PubMed] [Google Scholar]
- Bochman F. Quartz, silicosis and lung cancer: Meta analysis of the epidemiological studies. Proceedings of the 15th Symposium on Epidemiology; Copenhagen, Denmark. 2001. Available at: http://www.hvbg.de/e/bia/akt/archiv/ar2001/pdf/epicoh.pdf. [Google Scholar]
- Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421(3):691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- CDC. Center for Disease Control. Washington DC. Available at: http://www.cdc.gov
- Cohen B. 2005. Test of the linear no-threshold theory; rationale for procedures. Nonlinearity in Biology, Toxicology and Medicine (in press). [DOI] [PMC free article] [PubMed]
- Cole LA. 1993. Element of Risk: The Politics of Radon. AAAS Press.
- Curie M, Debierne A, Eve AS, et al. The Radioactive Constants as of 1930. Report to the International Radium-Standards Commission. Review of Modern Physics. 1931;3(3):427–445. (p 442). [Google Scholar]
- Deetjen P. Biologisch und therapeutische effekte niedrig dosierter ionisierender strahlung. Z Phys Med Baln Med Klim. 1990;19:5–102. [Google Scholar]
- Deetjen P, Jockel H. Gibs es noch Indikationen fur eine Therapie mit Radonbadern ? Internist Prax. 1992;32:353–355. [Google Scholar]
- Deetjen P. Scientific Principles of the Health Treatments in Bad Gastein and Bad Hofgastein. Sem Reports. Salzburg, Austria: University of Innsbrook; 1997. (ISSN 0256-4173). [Google Scholar]
- Draganic IG, Draganic ZD, Adloff JP. Radiation and Radioactivity on Earth and Beyond. Boca Raton, FL: CRC Press; 1990. pp. 3–7. [Google Scholar]
- Duport P. Is the radon risk overestimated? Neglected doses in the estimation of the risk of lung cancer in uranium underground miners. Radiat Prot Dosim. 2002;98:329–338. doi: 10.1093/oxfordjournals.rpd.a006724. [DOI] [PubMed] [Google Scholar]
- Erickson B. Radiation and Health: An Overview of Radon Therapy in the United States and Europe. Proceedings of the 14th Pacific Basin Nuclear Conference; Honolulu, HI. 2004. pp. 654–661. [Google Scholar]
- Franke A, Reiner L, Pratzel HG, et al. Long term efficacy of radon spa therapy in rheumatoid arthritis—a randomized, sham-controlled study and follow-up. Rheumatotology. 1997;39:894–902. doi: 10.1093/rheumatology/39.8.894. [DOI] [PubMed] [Google Scholar]
- Ghiassi-nejad M, Mortazavi SM, Cameron JR. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Physics. 2002;82:87–93. doi: 10.1097/00004032-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Hallenbeck WH. Radiation Protection. Boca Raton, FL: CRC Press; 1994. p. 14. [Google Scholar]
- Halliwell B. Free radicals, antioxidants and human disease: curiosity, cause or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Jackson R P J. Spas, Waters and Hydrotherapy in the Roman World. In: De Laine J and Johnston D (Eds). 1999. Roman Baths and Bathing. Part 1: Bathing and Society. J Roman Archaeol; Supplementary series No. 37: pp. 107–116.
- Jajte JM. Chemical-induced changes in intracellular redox state and in apoptosis. Int J Occupat Med Environ Health. 1997;10:203–212. [PubMed] [Google Scholar]
- Joss A, Kochanski JW, Karasek M. Radioterapia w chorobach naczyn obwodowych. Folia Medica Lodziensia. 2002;29:79–93. [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, et al. Residential Radon and Risk of Lung Cancer: A Combined Analysis of 7 North American Case-Control Studies. Epidemiology. 2005;2:137–145. doi: 10.1097/01.ede.0000152522.80261.e3. [DOI] [PubMed] [Google Scholar]
- Lubin LH, Boice JD. Lung cancer risk from residential radon. Meta-analysis of eight epidemiological studies. J NCI. 1997;89:49–57. doi: 10.1093/jnci/89.1.49. [DOI] [PubMed] [Google Scholar]
- Marcinowski F. Nationwide survey of residential radon levels in the U.S. Health Phys. 1992;62(6):S13. (Suppl) [Google Scholar]
- Marshalick BE, Fenko AN. The use of radon baths for rehabilitating the immune system of patients with bronchial asthma. VoprKurortol Fiziother LechFiz Kult. 1991;6:6–10. [PubMed] [Google Scholar]
- Marshalick BE, Shkolenko RL. The effect of radon therapy on the bacteriological and immunological indeces of patients with intestinal dysbacteriosis. VoprKurortol Fiziother LechFiz Kult. 1993;5:30–34. [PubMed] [Google Scholar]
- Mifune M, Kondo S, Tanooka, et al. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. J Japan Cancer Res. 1992;83:1. doi: 10.1111/j.1349-7006.1992.tb02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SZ. Multilevel mechanisms of stimulatory effect of low dose radiation on immunity. In: Sugahara T, Saga LA, Aoyama T, editors. Low Dose Irradiation and Biological Defense Mechanisms. The Netherlands: Elsevier Science Publishers; 1992. [Google Scholar]
- Ponikowska I, Adamczyk P, Vu Khai, et al. The Clinical Principles of Balneology & Physical Medicine. Balneology J—winter 2003. Adapted from: Ponikowska I. 2002. Polska droga rozwoju lecznictwa uzdrowiskowego. Balneologia Polska. 2002;44:105–113. Available in English at: http://www.amtamassage.org/journal/winter03_journal/balneology.html. [Google Scholar]
- Pratzel HG, Deetjen P. Radon in der Kurortmedizine. Geretsried, Germany: ISMH; 1997. [Google Scholar]
- Routh HB, Bhowmik KR, Parish LC, et al. Balneology, mineral water, and spas in historical perspective. Clin Dermatol. 1996;14:551–554. doi: 10.1016/s0738-081x(96)00083-1. [DOI] [PubMed] [Google Scholar]
- Soto J. Die Wirkung von Radon auf das Immunsystem [Effects of Radon on the Immune System] In: Pratzel HG, Deetjen P, editors. Radon in der Kurortmedizine. Geretsried, Germany: ISMH; 1997. [Google Scholar]
- Szerbin P. Natural radioactivity of certain spas and cave in Hungary. Environment Internat J. 1996;22(5):389–398. (Suppl). [Google Scholar]
- UNSCEAR (UN Scientific Committee on the Effect of Atomic Radiation) Sources and effects of ionizing radiation. New York: United Nations; 1993. [Google Scholar]
- UNSCEAR (UN Scientific Committee on the Effect of Atomic Radiation) Adaptive response to radiation in cells and organisms. New York: United Nations; 1994. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) Health Assessment Document for Diesel Engine Exhaust. Washington, DC: 2002. Report EPA/600-8-90/057F. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency) radon website link. Available at: http://www.epa.gov/iaq/radon
- Van Tubergen A, Landewe R, van dee Heijde, et al. Combined spa-exercise therapy is effective in patients with ankylosing spondylitis. Arthitis Rheum. 2001;45(5):430–438. doi: 10.1002/1529-0131(200110)45:5<430::aid-art362>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Van Tubergen A, van der Linden S. A brief history of spa therapy. Annals of the Rheumatic Diseases. 2002;61:273–275. doi: 10.1136/ard.61.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tubergen A, Hidding A. Spa and exercise treatment for ankylosing spondylitis—fact or fancy? Best Practice Res Clinic. Rheumatics. 2002;16(4):653–666. doi: 10.1053/berh.2002.0240. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Mitsunobu F, Kojima S, et al. The Elevation of p53 Protein Level and SOD Activity in the Residents Blood of the Misasa Radon Hot Spring District. J Radiat Res. 2005;46:21–24. doi: 10.1269/jrr.46.21. [DOI] [PubMed] [Google Scholar]
- Zdrojewicz Z, Belowska-Bien K. Radon i promieniowanie jonizujace a organism czlowieka [Radon and ionizing radiation in human body] Postepy Hig Med Dosw. 2004;58:150–157. English translation available from the corresponding author. Available on-line in Polish on http://www.phmd.pl/pub/phmd/vol_58/5275.pdf. [PubMed] [Google Scholar]
- Zdrojewicz Z, Belowska-Bien K. Ocena stezenia testosteronu u mezczyzn poddanych dzialaniu mediow radonowych [Estimating Testosterone Concentration in Male Patients Exposed to Radon Media] Adv Clin Exp Med. 2004;13(2):267–272. [Google Scholar]