Abstract
To understand the potential impact on risk from exposure to low-level ionizing radiation, we have investigated the modulation of gene expression, induction of DNA damage and of neoplastic transformation in human or rodent cells derived from cultures exposed in vitro to low dose γ-rays (a low linear energy transfer radiation) or very low fluences of α-particles (a high linear energy transfer radiation). Pre-exposure of cells to a low γ-ray dose protected cells from the DNA damaging and killing effects induced by a subsequent acute challenge exposure to γ-rays. Furthermore, a low dose chronic exposure to γ-rays decreased the frequency of micronucleus formation and neoplastic transformation to a level below the spontaneous rate in human and rodent cells respectively. In contrast, when cell cultures were exposed to low fluences of α-particles, wherein a small fraction of cells were irradiated, stressful effects were transmitted from the irradiated to adjoining nonirradiated bystander cells. The mechanisms underlying these effects and their relative contribution to the overall risk to ionizing radiation is discussed.
I. INTRODUCTION
Living organisms are continually exposed to low-level ionizing radiation (IR) from natural sources, with radon gas being the prime source. Due to its alpha-particle emitting decay products, radon gas has been considered to be the single largest naturally occurring environmental hazard(1). In addition, the human population has been increasingly subjected to low dose IR from activities related to deep space travel, nuclear technology, and the clean-up of sites that are contaminated with radioactive material. Perhaps, of greatest significance is exposure from diagnostic radiology, which has recognized an explosive growth in the past decade. Based on the current increase in computed tomography (CT) examinations, it is predicted that by the year 2010, one in every three individuals residing in economically developed countries will have a CT scan annually, with the likelihood of several repeats in the patient's lifetime(2, 3). As a result, there is a particular public and scientific interest in characterizing the biological effects of IR in the low dose/low fluence range at which these latter activities occur. Specifically, focus is on characterizing the underlying molecular and biochemical mechanisms.
Currently, for the purposes of radiation protection, the deleterious effects of radiation are assumed to have a linear dose response with no threshold. Furthermore, it is assumed that protracted (chronic) exposures require about twice the dose to cause the same effect as an acute exposure. The effects of sequential doses are assumed to be additive(4). One consequence of a linear, no threshold risk model is the assumption that exposure to any dose of radiation, however small, can potentially result in detrimental health effects. However, increasing in vitro and in vivo evidence in human and rodent systems shows that cellular exposure to doses as low as 0.01 Gy from low linear energy transfer (LET) radiation induces a protective mechanism that reduces the amount of chromosomal damage, and/or reduces or delays the onset of carcinogenesis caused by a subsequent exposure(5, 6). Importantly, exposure to doses below 0.1 Gy was shown, in some instances, to reduce the level of chromosomal damage due to endogenous oxidative processes(7). This phenomenon, termed adaptive response, has been shown to be dependent upon the dose rate, expression time, culture conditions, cell and tissue type, stage of the cell cycle, and the endpoint measured(8). The observation of adaptive responses in mammalian cells mirrors the evidence for the presence of radiation-inducible DNA repair systems in prokaryotes and lower eukaryotes(9), and hence supports the concept that its existence is evolutionarily conserved(10).
In contrast to radiation-induced adaptive responses, several studies, mainly in cell cultures exposed to high LET radiations such as α-particles, have shown that biological stress responses, including genetic effects, may be expressed in cells that received no radiation exposure. Such effects occur, presumably, as a result of signals transmitted from irradiated cells(11). Widespread experimental evidence now indicates that IR traversal through the nucleus of a cell is not a prerequisite to produce genetic damage or a biological response(12–14). Bystander cells in a population that are in the vicinity of directly targeted cells, or recipient of growth medium from irradiated cell cultures, have been shown to respond to the radiation exposure(15–18). Significant levels of genetic changes and lethality have been observed in bystander cells of varying genetic background, lineage, and organ origin, when such cells were in the neighborhood of cells targeted by α-particles.
While evidence for both adaptive and bystander effects has been well established, a clear understanding of the exact biochemical and molecular processes by which they occur remains unclear. Here, we describe aspects of our research focusing on characterizing the adaptive and bystander responses, and their underlying mechanisms, in confluent monolayer cultures of normal human or mouse cells exposed to low dose/low dose rate cobalt-60 γ-rays, or very low fluences of α-particles from a conventional broad-beam irradiator fitted with a plutonium-238 source.
II. ADAPTIVE RESPONSES TO γ-RAY EXPOSURES
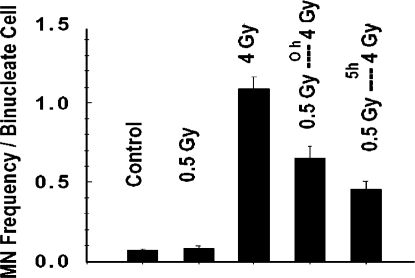
In early studies, we have tested the influence of low γ-ray doses delivered at low dose rate on expression of the adaptive response in density-inhibited AG1522 normal human skin fibroblast cultures. The endpoint of micronucleus (MN) formation was used as a measure of radiation-induced chromosomal breaks—micronuclei arise predominantly from un-rejoined DNA double–strand breaks (19) and have been strongly implicated in the process of cancer development in humans (20). The MN frequency in cells exposed to 0.5 Gy delivered at low dose rate (0.002 Gy/min), prior to being challenged by an acute 4 Gy dose, was significantly lower than in cells exposed to the challenge dose only (Figure 1). These results indicate that adapted cells are better protected against DNA damage that leads to chromosomal breaks. When a 5h incubation period at 37°C separated the priming and challenge doses, hence allowing more time for adaptive process(es) to be expressed and to operate, even less MN formation occurred following the challenge dose.
FIGURE 1.
Frequency of micronucleus formation in density-inhibited AG1522 normal human diploid fibroblast cultures exposed to γ-rays (0.5 Gy at 0.002 Gy/min and or 4 Gy at 1.8 Gy/min).
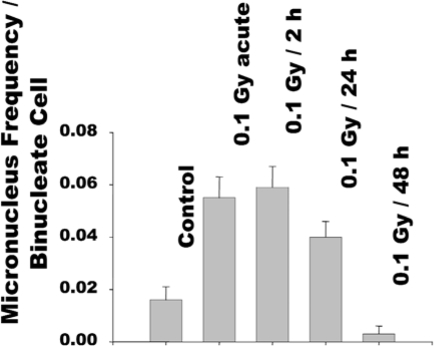
In more recent experiments, we have used human fibroblasts grown in a 3-dimensional architecture that mimics cell growth in vivo, and measured chromosomal damage and changes in expression of stress related proteins following cellular exposure to a single small dose (0.1 Gy) of γ-rays delivered at variable dose rates. Compared to sham-manipulated controls, a significant increase in MN occurred in cells exposed to 0.1 Gy delivered acutely (Figure 2). When the 0.1 Gy dose was protracted over 24h, the residual level of MN was significantly reduced. Importantly, when the dose was delivered over 48h, the MN frequency in the exposed cells was reduced to a level below the spontaneous rate. This pattern of MN formation correlated with the pattern of changes in phosphorylation of serine15 in the p53 protein. The p53 protein is activated and stabilized in response to a wide range of cellular stresses. Its activation is associated with phosphorylation of its serine15 residue. Similar to MN formation, an increase in serine15 phosphorylation occurred in cells exposed to acute 0.1 Gy. When the latter dose was protracted over 48 h, the level of serine15 phosphorylation was lower than detected in sham-manipulated control cells (data not shown). Both, the decrease in MN frequency and in serine-15 phosphorylation were associated with an increase in cellular content of the antioxidant glutathione(21), suggesting involvement of oxidative metabolism in expression of radiation-induced adaptive responses(22).
FIGURE 2.
Frequency of micronucleus formation in confluent AG1522 normal human fibroblast cultures exposed to 0.1 Gy from γ-rays at various dose rates(22).
Whereas the results in Figures 1 & 2 support the expression of radiation-induced protective mechanisms that result in reduced residual DNA damage in human cells exposed to low dose/low dose rate γ-radiation, for issues related to radiation protection, it is of interest to characterize the effect on carcinogenic risk. A model system suitable for studies of radiation-induced adaptive responses on carcinogenesis is the C3H 10T1/2 mouse embryo fibroblast “transformation assay”. In this assay, non-transformed cells in tissue culture can be transformed into demonstrably malignant cells by exposure to IR. When C3H 10T1/2 cells were challenged by a large acute γ-ray dose of 4 Gy, the transformation frequency was increased about 10-fold over the spontaneous frequency (Table 1). However, when cells were pre-exposed, 3.5 h earlier, to a priming dose of 0.1 Gy delivered at low dose rate (0.002 Gy/min), risk was not increased as predicted by the linear no threshold (LNT) hypothesis; it was actually decreased by 2- to 3-fold(23). These results (Table 1) mirror those described in Figure 1 with human cells, and support the induction by low dose γ-rays of protective mechanisms against radiation damage. The decrease in the transformation frequency was associated with a decrease in MN formation (Table 1), suggesting error-free repair of chromosomal damage. These results are thus inconsistent with the assumption that the cumulative cancer risk from two sequential exposures can never be less than one alone. They indicate that cells can adapt to small chronic IR exposures, and the adapted cells are better able to correctly repair lesions resulting from a subsequent exposure, and are less likely to be neoplastically transformed from that second exposure.
TABLE 1.
Pre-exposure to a chronic γ-ray adapting dose (0.1 Gy) reduces micronucleus formation and neoplastic transformation in confluent C3H 10T1/2 mouse embryo fibroblasts challenged by an acute γ-ray dose of 4 Gy(23).
| Treatment | Transformation frequency × 10−3 per viable cell (±SD) | Percentage of binucleated cells with micronuclei (±SD) |
|---|---|---|
| Control | 0.4 (0.4) | 11.5 (0.75) |
| 4 Gy | 4.1 (0.5) | 85.3 (2.30) |
| 0.1 Gy | 1.6 (0.7) | 16.2 (0.73) |
| 0.1 → 4 Gy | 2.2 (0.6) | 81.5 (1.99) |
→ indicates incubation at 37°C for 3.5 h.
In subsequent experiments, we examined the risk of neoplastic transformation induced by the small dose exposure alone(7). C3H 10T1/2 cells were irradiated with γ-ray doses from 0.001 to 0.1 Gy delivered chronically (0.002 Gy/min). They were incubated for 24 h after the exposure, following which they were assayed. Contrary to the predictions of the LNT model, which foresees that any dose of radiation, no matter how small, increases cancer risk, chronic exposure to doses from 0.001 to 0.1 Gy reduced the frequency of neoplastic transformation to a level below the spontaneous rate (Table 2). Statistical significance of these results was maintained when the data for the various radiation exposures were pooled and compared to control. However, when cells were assayed immediately after exposure, the transformation frequencies were not significantly different from the spontaneous frequency (data not shown), suggesting that time is needed for expression of radioprotective mechanisms (e.g. inducible DNA repair, antioxidation, induction of cell death).
TABLE 2.
The effect of low chronic (0.002 Gy/min) γ-ray doses on spontaneous transformation frequency(7).
| Treatment | Number of transformed foci/number of assay flasks | ρ Fisher exact test |
|---|---|---|
| Control | 46/85 | — |
| 0.001 Gy + 24 h holding | 5/27 | 2.4 × 10−2 |
| 0.01 Gy + 24 h holding | 5/42 | 7.8 × 10−4 |
| 0.1 Gy + 24 h holding | 6/41 | 2.4 × 10−3 |
| Summed data: 0.001+0.01+01 Gy with 24 h holding | 16/110 | 1.9 × 10−5 |
A dose of 0.001 Gy is approximately equivalent to the annual non-radon dose received from background radiation (but delivered more quickly in the experiments described in Table 2). Such dose is also in the range of a typical occupational exposure and represents, on average, about one track per cell that is hit(24), the lowest possible dose a cell can receive. Hence, the data in Table 2 imply that any single photon track through any one of these cells, whether from background radiation or other exposure, reduces the risk of spontaneous neoplastic transformation in that cell. These results therefore show that a single low dose of low LET radiation, in the background or occupational dose range, can in some circumstances induce processes, which reduce, rather than increase, the risk of neoplastic transformation. Since human cancer risks from exposure to high doses of IR have been well established, these results suggest that exposure of mammalian cells to low doses could induce molecular signaling processes that are different from those induced by high doses. This was recently confirmed by our observation that high and low dose γ-rays induce differential effects on mitochondrial membrane potential and protein import(25).
III. THE ALPHA-PARTICLE INDUCED BYSTANDER EFFECT
The IR-induced bystander effect has been broadly defined as the occurrence of biological effects in unirradiated cells as a result of exposure of other cells to IR(15,26). A bystander effect induced in cell cultures exposed to α-particles was initially described by Nagasawa and Little(12). An enhanced frequency of sister chromatid exchanges in 20–40% of cells was observed in Chinese hamster ovary fibroblast cultures exposed to fluences by which only 0.1–1% of the cells' nuclei were traversed by a particle track. These results indicated that the target for genetic damage by α-particles is much larger than the nucleus or in fact than the cell itself. This was subsequently confirmed by others for the same endpoint in human fibroblasts(27). Since, it has been shown that an enhanced frequency of specific gene mutations can also occur in bystander cells present in cultures exposed to very low fluences of α-particles(28,29). Also, an enhanced frequency of micronucleus formation and apoptosis in bystander cells was observed(30,31), and in vitro neoplastic transformation experiments have shown that bystander cells neighboring irradiated cells are also at risk(32). The latter studies thus suggest that, under some conditions, mutations and chromosomal aberrations induced in bystander cells may lead to tumorgenesis.
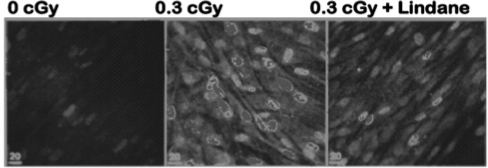
Using gene expression as an endpoint, it was also shown that stressful effects are transmittable from cells exposed to high LET IR to non-irradiated cells. It was found, by flow cytometry, that p53 levels were induced by α-particle irradiation in a greater fraction of cells than were hit by a particle track(33). We have further developed these studies and examined the modulation of stress sensitive proteins in a variety of human and rodent cell types using in situ immunodetection techniques(34). The data in Figure 3 describe the expression of the stress sensitive protein p21Waf1 in control and irradiated normal human fibroblast cultures—p21Waf1 is a p53 downstream effecter that regulates cellular growth, and whose expression is increased in cells that undergo DNA damage. Confluent density-inhibited cultures were exposed to a mean dose of 0.3 cGy from α-particles in the presence or absence of the gap-junction-inhibitor lindane—a chemical that disrupts the exchange of small molecules through gap-junction channels that link contiguous cells with each other. Based on microdosimetric calculations, at a mean dose of 0.3 cGy, about 2% of cells in the exposed culture would be traversed through the nucleus by an α-particle track. The data in Figure 3 indicate that in exposed cultures, a significantly greater cell fraction up-regulated p21Waf1. Interestingly, up-regulation of p21Waf1 occurred in aggregates of neighboring cells, supporting the view that damage signals were communicated from irradiated to bystander cells. This view was held up when the cultures were exposed to 0.3 cGy in the presence of lindane. The in situ immunofluorescence data in Figure 3 show that treatment of the exposed cultures with lindane resulted in inhibition of the aggregate pattern of p21Waf1 induction (Figure 3, right panel) that typically occurs in control irradiated cultures (Figure 3, mid panel). In irradiated cultures treated with lindane, p21Waf1 was induced primarily in single cells. Such cells were precisely identified through the use of CR-39-based culture dishes(35). These data thus implicate gap-junction intercellular communication in up-regulation of p21Waf1 in bystander cells present in cultures exposed to fluences of α-particles wherein a very small fraction of cell nuclei is traversed by a particle track.
FIGURE 3.
In situ immuno-detection of p21Waf1 in non-irradiated lindane-treated (40 μM), and irradiated AG1522 cultures exposed to 0.3 cGy α-particles in the presence or absence of lindane(34).
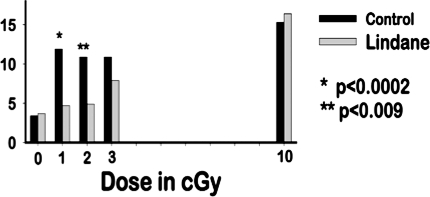
To investigate whether the bystander induction of p21Waf1 (Figure 3) is associated with higher levels of DNA damage than expected after irradiation of cell populations with low mean doses of α-particles, we measured the frequency of micronucleus formation in confluent cultures of AG1522 fibroblasts that were held in confluence for 3 h after the exposure. Compared to control non-exposed cultures, a 3-fold increase in the induction of micronuclei was detected after exposure to mean doses in the range of 1–3 cGy, and only a 4-fold increase after exposure to 10 cGy (Figure 4). At a mean dose of 10 cGy, 10-fold more cells in the population experience a nuclear traversal by an α-particle than by 1 cGy. Therefore, the magnitude of the response at low fluences suggests that non-traversed bystander cells were also subject to DNA damage. To investigate the involvement of gap-junction intercellular communication in the response, lindane was added to the cultures 2 h prior to exposure and remained for 3h thereafter. A highly significant reduction in the frequency of micronucleus formation was observed in cultures exposed to 1 or 2 cGy. At 10 cGy, lindane did not reduce the micronucleus frequency in confluent cultures exposed to this same mean dose (Figure 4). These data thus suggest that DNA damage may be the signal for the bystander induction of p21Waf1 in low fluence exposed confluent cell cultures. However, both effects may also be independent consequences of signals communicated from irradiated to bystander cells.
FIGURE 4.
Micronucleus formation in α-particle exposed confluent, density-inhibited AG1522 normal human fibroblast cultures. The cultures were irradiated in the presence or absence of the inter-cellular communication inhibitor lindane(34). P values were determined by the chi-square test.
The induction of micronuclei and the up-regulation of the stress sensitive p21Waf1 protein in bystander cells that neighbor α-particle irradiated cells is in contrast to the observations with low dose/low dose-rate γ-irradiated cell cultures (Figures 1 & 2 and Tables 1 & 2), whereby a γ-ray dose as little as 0.001 Gy induced a protective mechanism against endogenous damage or stressful effects from a subsequent challenge radiation exposure. If DNA damage were to occur in bystander cells in vivo and such damage persists and is transmitted to progeny cells, the observations in α-particle exposed cultures would significantly impact the assessment of cancer risk due to low level high LET IR exposures. However, the macroscopic dose from a single α-particle cell traversal is non-negligible (0.13 Gy in a AG1522 fibroblast)(36). Moreover, the specific energy in a directly hit area (e.g. nucleosome) may be equivalent to several Grays(37). In contrast, the dose deposited from a Compton electron is substantially smaller. Hence, further studies of the effects of radiation dose, dose-rate and LET in the propagation of protective or damaging responses may be critical in enhancing our understanding of low level IR risk.
IV. CONCLUSION
Some of the mechanisms (e.g. gap-junction intercellular communication, oxidative metabolism) that underlie the bystander effect have been also implicated in the adaptive response to IR and in some cases the same endpoint (e.g. cell death) has been used to examine expression of either phenomenon. However, classical adaptive response protocols involving low LET radiation are clearly distinct from those of bystander studies conducted mainly with high LET radiation. In the adaptive response, cells are pre-exposed to a small dose of low LET radiation prior to a challenge dose of the same type of radiation. In contrast, cells traversed by an α-particle receive a substantial dose (10–70 cGy) and undergo a complex type of DNA damage. While similar mediators may modulate the endpoint (e.g. viability) in both phenomena, the occurrence of opposite effects, such as pro-survival rather than cytotoxic effect, may reflect changes in concentration of the inducing factor(s). For example, reactive oxygen species have been shown to be a double-edged sword capable of inducing both proliferative or cell death effects depending on their concentration. Moreover, recent studies emphasized the effect of LET on the yield of water radiolysis products(38). Prevalence of different radiolysis species at the time of irradiation may induce dissimilar effects.
The bystander effect and adaptive response could also be mediated by distinct mechanisms/mediating factors. Induction of an adaptive response to low LET IR protected against bystander damage induced by α-particles(39). While, DNA damage was shown to be unequivocally induced in bystander cells, the adaptive response implicates the involvement of DNA repair, and up-regulation of antioxidation, which result in reduced residual DNA damage in exposed cells.
Human epidemiology alone has been unable to resolve the issue of whether there are low dose thresholds or whether there is an increased risk at low level ionizing radiation. Such inability does not mean that these effects do not occur in vivo. In vitro mechanistic studies in model cell systems provide a unique opportunity to control confounding factors, and address, in controlled studies, the relevance to risk of exposure to chronic low dose/low LET radiation, or to low fluences of high LET energetic particles.
ACKNOWLEDGMENT
We are grateful to Dr. John B. Little, Dr. Ronald E.J. Mitchel and Dr. G. Peter Raaphorst for their mentorship and continuous guidance. We also thank our colleagues at the New Jersey Medical School for many stimulating discussions and for their support. Research Grants FG02-02ER63447 from the U.S. Department of Energy and 1RO1-CA92262-01A1 from the National Institutes of Health partially funded this investigation.
REFERENCES
- 1.BEIR-VI Health Effects of Exposure to Radon (BEIR VI) Washington, D.C.: National Academy Press; 1998. [PubMed] [Google Scholar]
- 2.Brenner D.J., Elliston C.D., Hall E.J., Berdon W.E. Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: comment on “point/counterpoint: in x-ray computed tomography, technique factors should be selected appropriate to patient size. against the proposition”. Med Phys. 2001;28:2387–2388. doi: 10.1118/1.1415074. [DOI] [PubMed] [Google Scholar]
- 3.Baker S.R. Musings at the beginning of the hyper-CT era. Abdominal Imaging. 2003;28:110–114. doi: 10.1007/s00261-002-0063-x. [DOI] [PubMed] [Google Scholar]
- 4.UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources, Effects, and Risks of Ionizing Radiation. The 1988 Report to the General Assembly with Annexes. New York: United Nations, 1988.
- 5.Wolff S. Failla Memorial Lecture. Is radiation all bad? The search for adaptation. Radiat Res. 1992;131:117–123. [PubMed] [Google Scholar]
- 6.Mitchel R.E., Jackson J.S., McCann R.A., Boreham D.R. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- 7.Azzam E.I., de Toledo S.M., Raaphorst G.P., Mitchel R.E. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- 8.Shadley J.D. Chromosomal adaptive response in human lymphocytes. Radiat Res. 1994;138:S9–12. [PubMed] [Google Scholar]
- 9.Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977;267:281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- 10.Mitchel R.E., Azzam E.I., de Toledo S.M. Adaptation to Ionizing Radiation in Mammalian Cells. In: Koval T.M., editor. Stress-Inducible Processes in Higher Eukaryotic Cells. New York: Plenum Press; 1997. pp. 221–243. [Google Scholar]
- 11.Azzam E.I., de Toledo S.M., Little J.B. Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets. 2004;4:53–64. doi: 10.2174/1568009043481641. [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa H., Little J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 13.Wu L.J., Randers-Pehrson G.R., Xu A., Waldren C.A., Geard C.R., Yu Z.L., Hei T.K. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc. Natl. Acad. Sci. USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao C., Folkard M., Michael B.D., Prise K.M. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci U S A. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little J.B., Azzam E.I., de Toledo S.M., Nagasawa H. Bystander effects: intercellular transmission of radiation damage signals. Radiat Prot Dosimetry. 2002;99:159–162. doi: 10.1093/oxfordjournals.rpd.a006751. [DOI] [PubMed] [Google Scholar]
- 16.Azzam E.I., de Toledo S.M., Little J.B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 17.Morgan W.F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Mothersill C., Seymour C.B. Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 19.Heddle J.A., Carrano A.V. The DNA content of micronuclei induced in mouse bone marrow by gamma-irradiation: evidence that micronuclei arise from acentric chromosomal fragments. Mutat Res. 1977;44:63–69. doi: 10.1016/0027-5107(77)90115-4. [DOI] [PubMed] [Google Scholar]
- 20.Karran P. DNA double strand break repair in mammalian cells. Curr Opin Genet Dev. 2000;10:144–150. doi: 10.1016/s0959-437x(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 21.Biaglow J.E., Varnes M.E., Epp E.R., Clark E.P., Tuttle S.W., Held K.D. Role of glutathione and other thiols in cellular response to radiation and drugs. Drug Metab Rev. 1989;20:1–12. doi: 10.3109/03602538908994142. [DOI] [PubMed] [Google Scholar]
- 22.De Toledo, S. M., Asaad, N., Venkatachalam, P., Li, L., Spitz, D. R., and Azzam, E. I. Adaptive responses to low dose/low dose-rate gamma in normal human fibroblasts: The role of growth architecture and oxidative metabolism. Radiat Res, in press, 2006. [DOI] [PubMed]
- 23.Azzam E.I., Raaphorst G.P., Mitchel R.E. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat Res. 1994;138:S28–31. [PubMed] [Google Scholar]
- 24.Bond V.P., Feinendegen L.E. What is a ‘low dose' of radiation? Int. J. Radiat. Biol. 1988;53:1–12. doi: 10.1080/09553008814550361. [DOI] [PubMed] [Google Scholar]
- 25.Pandey B.N., Gordon D.A., Pain D., Azzam E.I. Normal human fibroblasts exposed to high or low dose ionizing radiation: Differential effects on mitochondrial protein import and membrane potential. Antioxid Redox Signal. 2006;8:1253–1261. doi: 10.1089/ars.2006.8.1253. [DOI] [PubMed] [Google Scholar]
- 26.Mothersill C., Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande A., Goodwin E.H., Bailey S.M., Marrone B.L., Lehnert B.E. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts—Evidence for an extranuclear target. Radiat. Res. 1996;145:260–267. [PubMed] [Google Scholar]
- 28.Nagasawa H., Little J.B. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle irradiation: Evidence for a bystander effect. Radiat. Res. 1999;152:552–557. [PubMed] [Google Scholar]
- 29.Zhou H.N., Randers-Pehrson G., Hei T.K. Studies of bystander mutagenic response using a charged particle microbeam. Radiation Research. 2000;153:234–235. [Google Scholar]
- 30.Prise K.M., Belyakov O.V., Folkard M., Micheal B.D. Studies on bystander effects in human fibroblasts using a charged particle microbeam. Int. J. Radiat. Biol. 1998;74:793–798. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- 31.Azzam E.I., de Toledo S.M., Spitz D.R., Little J.B. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 32.Sawant S.G., Randers-Pehrson G., Geard C.R., Brenner D.J., Hall E.J. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Hickman A.W., Jaramillo R.J., Lechner J.F., Johnson N.F. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994;54:5797–5800. [PubMed] [Google Scholar]
- 34.Azzam E.I., de Toledo S.M., Little J.B. Direct evidence for the participation of gap-junction mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaillard, S., Pusset, D., De Toledo, S. M., Azzam, E. I., and Fromm, M. Distance distribution of bystander effects in alpha-particle irradiated cell populations using a CR-39-based culture dish. Radiat Meas, In press, 2006.
- 36.Neti P.V., de Toledo S.M., Perumal V., Azzam E.I., Howell R.W. A multi-port low-fluence alpha-particle irradiator: fabrication, testing and benchmark radiobiological studies. Radiat Res. 2004;161:732–738. doi: 10.1667/rr3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cucinotta F.A., Nikjoo H., Goodhead D.T. Model for radial dependence of frequency distributions for energy imparted in nanometer volumes from HZE particles. Radiat Res. 2000;153:459–468. doi: 10.1667/0033-7587(2000)153[0459:mfrdof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Jay-Gerin J.P., Meesungnoen J., Banville P., Mankhetkorn S. Comment on “The radiation-induced lesions which trigger the bystander effect”. Mutat Res. 2003;525:125–127. doi: 10.1016/s0027-5107(03)00002-2. by J.F. Ward [Mutat. Res. 499 (2002) 151–154] [DOI] [PubMed] [Google Scholar]
- 39.Sawant S.G., Randers-Pehrson G., Metting N.F., Hall E.J. Adaptive response and the bystander effect induced by radiation in C3H 10T(1/2) cells in culture. Radiat Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]