Abstract
There are a number of studies that show radiation can cause heritable mutations in the offspring of irradiated organisms. These “germ-line mutations” have been shown to occur in unique sequences of DNA called “minisatellite loci”. The high frequencies of spontaneous and induced mutations at minisatellite loci allow mutation induction to be measured at low doses of exposure in a small population, making minisatellite mutation a powerful tool to investigate radiation-induced heritable mutations. However, the biological significance of these mutations is uncertain, and their relationship to health risk or population fitness is unknown. We have adopted this mutation assay to study the role of adaptive response in protecting mice against radiation-induced heritable defects. We have shown that male mice, adapted to radiation with a low dose priming exposure, do not pass on mutations to their offspring caused by a subsequent large radiation exposure to the adapted males. This presentation and paper provide a general overview of radiation-induced mutations in offspring and explain the effect of low dose exposures and the adaptive response on these mutations.
It is also known that exposure of pregnant females to high doses of radiation can cause death or malformation (teratogenesis) in developing fetuses. Malformation can only occur during a specialized stage of organ formation known as organogenesis. Studies in rodents show that radiation-induced fetal death and malformation can be significantly reduced when a pregnant female is exposed to a prior low dose of ionizing radiation. The mechanism of this protective effect, through an adaptive response, depends on the stage of organogenesis when the low dose exposures are delivered. To better understand this process, we have investigated the role of an important gene known as p53. Therefore, this report will also discuss fetal effects of ionizing radiation and explain the critical stages of development when fetuses are at risk. Research will be explained that investigates the biological and genetic systems (p53) that protect the developing fetus and discuss the role of low dose radiation adaptive response in these processes.
I. INTRODUCTION
There are a number of negative health concerns associated with exposure to ionizing radiation. In humans, the most important issues related to high dose exposures include: 1) immediate death (hours to months) due to acute radiation syndromes of the central nervous system, gastrointestinal system or hematopoietic system; 2) later somatic effects such as cancer induction due to transformation of normal cells; or 3) reproductive effects such as fetal malformation or mutations in germ-line cells (reproductive tissue) that could be inherited in the next generation of offspring. This paper focuses on the latter topic and relates to two major issues associated with radiation effects on reproduction (heritable mutations and fetal malformation) and how radiation risks associated with these endpoints may be modified by the adaptive response. However, mutations in ESTR DNA have not been related to functional changes that have biological or evolutionary significance. These changes may simply provide a reflection of radiation-induced changes that are capable of being transmitted to the offspring that may or may not correlate to some biological risk.
Heritable Mutations
A number of studies have demonstrated adaptive response at the whole-animal level in vertebrates. Some of the documented positive effects of pre-irradiation have included: reduced short-term mortality rates in mice,1–4 reduced frequency of chromosome abnormalities in mice5,6 and humans7–9, and reduced risk of cancer development in mice.10–12 These studies provide clear evidence for reduction and/or delay of genetic instability and ultimately disease through adaptive response; however, the majority of both in vivo and in vitro studies have focused on somatic markers of DNA damage or cancer. An area of research that has received much less attention is the modification of risk associated with radiation-induced heritable mutations through adaptive response in mammalian germ cells.
Mouse expanded simple tandem repeat (ESTR) DNA loci are efficient genetic markers for studying heritable mutation processes.13–15 ESTRs consist of long tandem arrays of 4 to 6-base pair repeat units that are unstable in the germ line and mutate primarily by insertion or deletion of a number of repeat units. Mutations of this kind can be detected in the progeny of experimental animals by pedigree DNA profiling using single and multilocus DNA fingerprinting.16–18 Previous studies on ionizing radiation have shown that ESTR mutations can be induced in the germ line of male mice in a dose dependent manner during pre-meiotic stages of spermatogenesis, and that the doubling dose for ESTR mutation induction is similar to that obtained using the specific locus test.13 In this report we show that an adaptive response to ionizing radiation reduces the induction of germ line mutations at ESTR loci in the paternal germ line of mice.
Fetal Malformation
The p53 tumor suppressor protein is known to regulate growth arrest and apoptosis in response to DNA damage, and has been shown to respond to ionizing radiation in fetal murine tissues in a manner that is both tissue and gestational time dependent.19 Exposure of the murine fetus to large doses of radiation during the in utero period of organogenesis results in malformations. The extent of the malformations is dose dependent but exhibits an apparent threshold at about 30–50 cGy.20 That threshold has recently been shown to be dose rate sensitive, increasing with decreasing dose rate.21 Wang et al. have linked these malformations to radiation-induced apoptosis.22 They have reported that mice exposed to 5 Gy of X rays in utero on day 11 of gestation showed an increase in the number of apoptotic cells in the predigital regions in the forelimb buds, detected 4 h after irradiation. That apoptotic response correlated with teratogenic anomalies in the limbs of the fetuses subsequently observed on gestational day 19. Susceptibility to radiation-induced apoptosis in the predigital regions and the resulting digital defects depended on Trp53 status, with Trp53 (+/+) mice the most sensitive, Trp53 (+/−) intermediate, and Trp53 (−/−) the most resistant.22 The reduction in teratogenesis observed with decreased dose rate has also been shown to depend on functional Trp53 in mice.21
The work described here examined the adaptive effect of low dose exposure on radiation induced appendage teratogenesis in murine fetuses. The experiments tested the influence of fetal Trp53 status (normal, heterozygous or null) and the influence of gestational time on the ability of a low dose to modify the teratogenic effect of a subsequent large dose. The exposures were given during the period of fetal organogenesis and the teratogenic effect discussed here is tail shortening. The full scientific paper describing the teratogenic effects of high doses and the adaptive response on tail shortening and digit malformation during various gestational stages has been recently published.23
II. METHODS
Heritable Mutations
The mice used in the mutation studies were an outbred Swiss-Webster strain from a specific pathogen-free colony (Taconic Breeding Laboratories, Germantown, NY, USA). There were four treatment groups of 10 males (7 to 9 weeks old, 33 to 40 g) (i) 0 Gy (sham-irradiated control), (ii) 0.5 Gy, (iii) 1.0 Gy, and (iv) 0.1 + 1.0 Gy given acute doses of whole-body ionizing radiation from a broad-beam cesium-137 source. Animals in the control group were sham irradiated by being placed in the Cs-137 unit, but the source was not exposed. Mice in the 0.5 and 1.0 Gy groups received one acute dose, whereas those in the 0.1 + 1.0 Gy group received an adapting dose of 0.1 Gy, followed by a larger challenge dose of 1.0 Gy 24 hours later. All radiation doses were delivered at 0.33 Gy per minute. Dosimetry was performed using a pre-calibrated Farmer dosimeter with a 0.6 cm3 chamber. The chamber was calibrated at the National Research Council of Canada Laboratory.
Irradiated males were bred to untreated females, 9 weeks post-irradiation. This delay in breeding was selected to ensure that fertilizations resulted from mature sperm that developed from irradiated 2n-sper-matogonial stem cells.13 Males were euthanized 14 days post-pairing, and cardiac tissue collected for DNA extraction. Females and all pups were euthanized at 96 hours following delivery, and cardiac tissue from females and tail / hind-end tissues from pups were collected for DNA extraction. The McMaster University Animal Research Ethics Board following the guidelines of the Canadian Council on Animal Care approved all animal procedures.
The approach used to detect germ line mutations at ESTR loci has been described elsewhere.24 Mutations were observed as bands in offspring that deviated by 1.0 mm or more relative to their parental progenitors based on comparison to the in-lane size standard.25 Mutation scoring was performed without knowledge of treatment group, and verified by an independent observer.
Fetal Malformation
Male mice carrying a single defective copy of the Trp53 gene (C57Bl/6J-Trp53tm1Tyj) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and were crossed with 129X1/SvJ female mice (Trp53 +/+) also obtained from the Jackson Laboratory. The resulting F1 progeny were genotyped and the male and female Trp53 heterozygotes crossed to produce pregnant females bearing all three Trp53 genotypes, normal (Trp53 +/+), heterozygous (Trp53 +/−) and null (Trp53 −/−). A timed breeding protocol was used to control the extent of gestation at the time of radiation treatment.23 Female mice, pregnant at either day 10 or day 11 of gestation, were exposed to 30 cGy of 60Co gamma radiation (GammaCell 200, Atomic Energy of Canada Limited) at a dose rate of about 0.85 cGy/s while housed in a container designed to maintain the animal facility specific pathogen free conditions. At the end of irradiation, the animals were returned to normal housing. Control, unirradiated animals were transported to but not placed in the irradiator. Twenty-four hours after this low dose exposure, these animals and the unexposed controls were further exposed to 4 Gy of 60Co gamma radiation again while housed in a container designed to maintain the specific pathogen-free animal facility conditions.26 Some other control animals received neither irradiation. These unirradiated animals were transported to but not placed in the irradiator. At the end of irradiation the animals were returned to normal housing until gestation was terminated on day 18 by euthanization of the pregnant females and subsequent surgical removal of the fetuses. Fetal mice were genotyped, after being removed from the female by caesarean section on day 18 of gestation, by a tail clip protocol previously described.23 Fetal tail length was used as an indicator of malformation and was measured to the nearest 0.5 mm.
III. RESULTS
Heritable Mutations
Single and multilocus ESTR probes detected an overall elevation in germ line mutation frequency of more than two-fold in 0.5 and 1.0 Gy irradiated groups compared to the control (Figure 1a, 1b). The relative elevation above the control was similar in both of these groups despite the increase in radiation dose. In contrast, a smaller, non-significant elevation in mutation frequency above the control was detected with single locus ESTR probes in the 0.1 + 1.0 Gy group, and no apparent elevation with multilocus probe MMS10 (Figure 1a, 1b).
FIGURE 1.
Overall germ line mutation rates at ESTR loci in groups of mice where males were treated with four different doses of ionizing radiation from a cesium-137 source. Mutation rates were determined using (a) single locus markers Ms6-hm and Hm-2 pooled, and (b) multilocus probe MMS10. Asterisks indicate a significant difference from the control group after Bonferroni correction.
Fetal Malformation
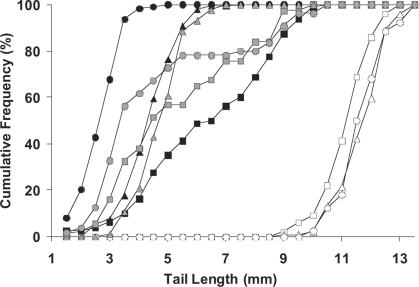
As reported previously,27,28 a 4 Gy radiation exposure on day 11 of gestation had a greater teratogenic effect on the fetal mice than the same exposure on day 12. This greater effect was seen as a significantly greater tail shortening (Figure 2) in all three Trp53 genotypes. We reported previously that at both gestational times the greatest teratogenic effect was seen in the Trp53 normal animals and the least effect in the Trp53 null fetuses, with the Trp53 heterozygotes being intermediate.27 Exposure of the fetuses to 30 cGy, 24 h prior to a 4 Gy exposure on day 11 of gestation significantly changed the teratogenic effect of the 4 Gy exposure. The prior 30 cGy exposure produced a protective effect in the Trp53 normal fetuses, resulting in a significant shift in tail length distribution toward longer tails (p < 10−4, Figure 2) compared to the Trp53 normal fetuses that received only the 4 Gy exposure. In the Trp53 heterozygous fetuses, there was a small but significant protective effect of the prior exposure mean tail length and the distribution of tail lengths (p < 0.04, Figure 2). The 30 cGy prior exposure did not protect the Trp53 null fetuses, but resulted in increased teratogenic effects, with mean tail length significantly reduced and tail length distribution significantly shifted toward shorter tails (p < 0.05, Figure 2).
FIGURE 2.
The influence of a low dose given on gestational day 10, on the tail length of fetal mice exposed to 4 Gy on gestational day 11. The figure shows the influence of Trp53 genotype on the cumulative frequency of fetuses with the indicated tail length as measured on day 18 of gestation. Open symbols represent unirradiated control fetuses; closed symbols represent fetuses exposed to 4 Gy on day 11 of gestation; shaded symbols represent fetuses exposed to 30 cGy, 24 h prior to 4 Gy irradiation on day 11 of gestation. Circles, Trp53 +/+; triangles, Trp53 +/−; squares, Trp53 −/−.
IV. DISCUSSION
Heritable Mutations
We irradiated male mice during spermatogonial stem cell stages of spermatogenesis, and found that germ line ESTR mutation rates did not increase in a dose-dependent manner. Males in the adapted group (0.1 + 1.0 Gy) received the highest total radiation dose, but their offspring inherited ESTR mutations at lower frequencies than those in the 0.5 or 1.0 Gy groups. This pattern, where a small prior radiation dose attenuates the effects of a subsequent larger dose, is indicative of adaptive response.
Our findings provide evidence that the modifying effects of adaptive response in the mammalian germ line extend beyond gross chromosomal abnormalities in germ cells29, 30 or dominant lethal mutations31 and reduce the risk of transmissible genetic mutations to viable offspring.
At this point it is not clear whether our findings apply to risk assessment for heritable mutations in humans exposed to ionizing radiation, but there are some interesting possibilities. Further study of the effects of dose fractionation, dose rate and adaptive response on germ line mutation rates at repetitive DNA loci is required to test this hypothesis, and to provide more information about potential mechanisms.
Repetitive DNA markers have been used effectively to detect radiation-induced heritable mutations in the laboratory using mice13, 32, and in natural populations of humans, birds, and plants living near radioactive contamination sites.33–37 The potential value of this class of genetic marker for health risk assessment in radiobiology has recently been recognized (e.g., Ref. 38), however, little is known about the utility of this approach for the study of biological processes that modify risk, such as adaptive response, hormesis, or bystander effect. [For an exception, see multigenerational mouse studies, which show a bystander effect that transcends generations.39, 40] Our results suggest that adaptive response reduces the frequency of mutation events at ESTR loci in the mammalian germ line, and that ESTR markers may be a suitable genetic system for further investigation of biological radiation risk modifiers.
Fetal Malformation
Wang et al. have reported that a window exists for the ability of a low dose to produce an adaptive response and protect the fetus against the teratogenic effects of a subsequent exposure.41 They presented evidence that a 30 cGy priming dose on gestational day 11 reduced malformations (mainly limb defects and short tails) resulting from a second exposure of 5 Gy given one day later. We have further examined the window of effect for this adaptive response and compared the response in fetuses of varying Trp53 status. Our results show qualitatively similar windows in time of effect of the low priming dose, but we show that these effects vary with the Trp53 status of the fetus. As reported by Wang et al.,41 our data show that a low dose can protect normal (Trp53 +/+) fetuses from tail shortening resulting from a large (4 Gy) exposure given 24 h later. In our strain of mice, that protection occurred when the priming dose was given on gestational day 10. Qualitatively, the same protective effect of a day 10 low dose exposure on tail shortening was seen in the Trp53 heterozygous (+/−) fetuses, although the magnitude of the protective effect was much reduced. These results suggest that, in the presence of functional Trp53 dependent apoptosis, low doses show a window for protection against the teratogenic effects of a high dose, but, as the level of functional Trp53 decreases, the ability of a low dose to protect also diminishes or vanishes. In the absence of functional Trp53, the effect of a prior low dose was very different. Given on gestational day 10, 24 h before a 4 Gy dose on day 11, the prior low dose resulted in a clear increase in tail teratogenesis in Trp53 null fetuses.
These results indicate that adaptive protection on day 10 of gestation against radiation induced teratogenesis on day 11 of gestation in tails resulted from a Trp53 dependent process. We conclude that prior exposure to low doses can protect against teratogenesis resulting from the Trp53 dependent process, although the protection shows a gestational time window for the effect.
ACKNOWLEDGMENTS
Funding for this research was provided by the Toxic Substances Research Initiative, the Natural Sciences and Engineering Research Council of Canada and the CANDU Owners Group.
REFERENCES
- 1.Cronkite E.P., Sipe C.R., Eltzholtz D.C., Chapman W.H., Chambers F.W., Jr Increased tolerance of mice to lethal X-radiation as a result of previous sublethal exposures. Proc. Soc. Exp. Biol. Med. 1950;73:184–187. [Google Scholar]
- 2.Dacquisto M.P. Acquired radioresistance – a review of the literature and report of a confirmatory experiment. Radiat. Res. 1959;10:118–129. [PubMed] [Google Scholar]
- 3.Yonezawa M., Misonoh J., Hosokawa Y. Two types of X-ray-induced radioresistance in mice: presence of 4 dose ranges with distinct biological effects. Mutat. Res. 1996;358:237–243. doi: 10.1016/s0027-5107(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 4.Nose M., Wang B., Itsukaichi H., Yukawa O., Hayata I., Yamada T., Ohyama H. Rescue of lethally irradiated mice from hematopoietic death by pre-exposure to 0.5 Gy X-rays without recovery from peripheral blood cell depletion and its modification by OK432. Radiat. Res. 2001;156:195–204. doi: 10.1667/0033-7587(2001)156[0195:rolimf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Wojcik A., Tuschl H. Indications of an adaptive response in C57BL mice pre-exposed in vivo to low doses of ionizing radiation. Mutat. Res. 1990;243:67–73. doi: 10.1016/0165-7992(90)90125-4. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi Z., Kesavan P.C. Low-dose radiation-induced adaptive response in bone marrow cells of mice. Mutat. Res. 1993;302:83–89. doi: 10.1016/0165-7992(93)90008-j. [DOI] [PubMed] [Google Scholar]
- 7.Gourabi H., Mozdarani H. A cytokinesis-blocked micronucleus study of the radioadaptive response of lymphocytes of individuals occupationally exposed to chronic low doses of radiation. Mutagenesis. 1998;13:475–480. doi: 10.1093/mutage/13.5.475. [DOI] [PubMed] [Google Scholar]
- 8.Monsieurs M.A., Thierens H.M., Vral A.M., Van De Wiele C., De Ridder L.I., Dierckx R.A. Adaptive responses in patients treated with 131I. J. Nucl. Med. 2000;41:17–22. [PubMed] [Google Scholar]
- 9.Thierens H., Vral A., Barbe M., Meijlaers M., Baeyens A., DeRidder L. Chromosomal radiosensitivity study of temporary nuclear workers and the support of the adaptive response induced by occupational exposure. Int. J. Radiat. Biol. 2002;78:1117–1126. doi: 10.1080/0955300021000034710. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharjee D. Role of radioadaptation on radiation-induced thymic lymphoma in mice. Mutat. Res. 1996;358:231–235. doi: 10.1016/s0027-5107(96)00125-x. [DOI] [PubMed] [Google Scholar]
- 11.Mitchel R.E.J., Jackson J.S., McCann R.A., Boreham D.R. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat. Res. 1999;152:273–279. [PubMed] [Google Scholar]
- 12.Mitchel R.E.J., Jackson J.S., Morrison D.P., Carlisle S.M. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat. Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Dubrova Y.E., Plumb M., Brown J., Fennelly J., Bois P., Goodhead D., Jeffreys A.J. Stage specificity, dose response, and doubling dose for mouse minisatellite germline mutation induced by acute radiation. Proc. Natl. Acad. Sci., USA. 1998;95:6251–6255. doi: 10.1073/pnas.95.11.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubrova Y.E., Plumb M., Brown J., Boulton E., Goodhead D., Jeffreys A.J. Induction of minisatellite mutations in the mouse germline by low-dose chronic exposure to gamma radiation and fission neutrons. Mutat. Res. 2000;453:17–24. doi: 10.1016/s0027-5107(00)00068-3. [DOI] [PubMed] [Google Scholar]
- 15.Barber R., Plumb M., Boulton E., Roux I., Dubrova Y.E. Elevated mutation rates in the germ line of first and second-generation offspring of irradiated male mice. Proc. Natl. Acad. Sci. USA. 2002;99:6877–6882. doi: 10.1073/pnas.102015399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly R., Bulfield G., Collick A., Gibbs M., Jeffreys A.J. Characterization of a highly unstable mouse minisatellite locus: evidence for somatic mutation during early development. Genomics. 1989;5:844–856. doi: 10.1016/0888-7543(89)90126-2. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs M., Collick A., Kelly R., Jeffreys A.J. A tetranucleotide repeat mouse minisatellite displaying substantial somatic instability during early preimplantation development. Genomics. 1993;17:121–128. doi: 10.1006/geno.1993.1292. [DOI] [PubMed] [Google Scholar]
- 18.Bois P., Williamson J., Brown J., Dubrova Y.E., Jeffreys A.J. A novel unstable mouse VNTR family expanded from SINE B1 elements. Genomics. 1998;49:122–128. doi: 10.1006/geno.1998.5228. [DOI] [PubMed] [Google Scholar]
- 19.MacCallum D.E., Hupp T.R., Midgley C.A., Stuart D., Campbell S.J., Harper A., Walsh F.S., Wright E.G., Balmain A., Lane D.P., Hall P.A. The p53 response to ionising radiation in adult and developing murine tissues. Oncogene. 1996;13:2575–2587. [PubMed] [Google Scholar]
- 20.Hossain M., Devi P.U., Bisht K.S. Effect of prenatal gamma irradiation during the late fetal period on the postnatal development of the mouse. Teratology. 1999;59:133–138. doi: 10.1002/(SICI)1096-9926(199903)59:3<133::AID-TERA4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Kato F., Ootsuyama A., Nomoto S., Kondo S., Norimura T. Threshold effect for teratogenic risk of radiation depends on dose-rate and p53-dependent apoptosis. Int. J. Radiat. Biol. 2001;77:13–19. doi: 10.1080/09553000010001899. [DOI] [PubMed] [Google Scholar]
- 22.Wang B., Fujita K., Ohhira C., Watanabe K., Odaka T., Mitani H., Hayata I., Ohyama H., Yamada T., Shima A. Radiation-induced apoptosis and limb teratogenesis in embryonic mice. Radiat. Res. 1999;151:63–68. [PubMed] [Google Scholar]
- 23.Mitchel R.E.J, Dolling J-A, Misonoh J., Bahen M.E., Boreham D.R. Influence of Prior Exposure to Low Dose Adapting Radiation on Radiation-Induced Teratogenic Effects in Fetal Mice with Varying Trp53 Function. Radiation Res. 2002;158(4):458–463. doi: 10.1667/0033-7587(2002)158[0458:iopetl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Somers C.M., Yauk C.L., White P.A., Quinn J.S. Air pollution induces heritable DNA mutations. Proc. Natl. Acad. Sci. USA. 2002;99:15904–15907. doi: 10.1073/pnas.252499499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yauk C.L., Dubrova Y.E., Grant G.R., Jeffreys A.J. A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutat. Res. 2002;500:147–156. doi: 10.1016/s0027-5107(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 26.McCann R.A., Wyatt H.M., Smith B.P., Carlisle S.M. A simple transport system for radiation treatment of specific pathogen-free mice in lifetime studies. Contemp. Top. Lab. Anim. Sci. 2001;40:39–42. [PubMed] [Google Scholar]
- 27.Boreham D.R., Dolling J-A., Misonoh J., Mitchel R.E.J. Radiation Induced Teratogenic Effects in Fetal Mice With Varying Trp 53 Function: Influence of Prior Heat Stress. Radiat. Res. 2002;158:449–457. doi: 10.1667/0033-7587(2002)158[0449:riteif]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Boreham D.R., Dolling J-A., Misonoh J., Mitchel R.E.J. Teratogenic Effects of Mild Heat Stress During Mouse Embryogenesis: Effect of Trp53. Radiat. Res. 2002;158:443–448. doi: 10.1667/0033-7587(2002)158[0443:teomhs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Cai L., Liu S.-Z. Induction of cytogenetic adaptive response of somatic and germ cells in vivo and in vitro by low-dose X-irradiation. Int. J. Radiat. Biol. 1990;58:187–194. doi: 10.1080/09553009014551541. [DOI] [PubMed] [Google Scholar]
- 30.Cai L., Jiang J., Wang B., Yao H., Wang X. Induction of an adaptive response to dominant lethality and to chromosome damage of mouse germ cells by low dose radiation. Mutat. Res. 1993;303:157–161. doi: 10.1016/0165-7992(93)90017-p. [DOI] [PubMed] [Google Scholar]
- 31.Cai L., Wang P., Piao X.-G. Cytogenetic adaptive response with multiple small x-ray doses in mouse germ cells and its biological influence on the offspring of adapted males. Mutat. Res. 1994;324:13–17. doi: 10.1016/0165-7992(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 32.Dubrova Y.E., Jeffreys A.J., Malashenko A.M. Mouse minisatellite mutations induced by ionizing radiation. Nat. Genet. 1993;5:92–94. doi: 10.1038/ng0993-92. [DOI] [PubMed] [Google Scholar]
- 33.Dubrova Y.E., Nesterov V.N., Krouchinsky N.G., Ostapenko V.A., Neumann R., Neil D.L., Jeffreys A.J. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683–686. doi: 10.1038/380683a0. [DOI] [PubMed] [Google Scholar]
- 34.Dubrova Y.E., Nesterov V.N., Krouchinsky N.G., Ostapenko V.A., Vergnaud G., Giraudeau F., Buard J., Jeffreys A.J. Further evidence for elevated human minisatellite mutation rate in Belarus eight years after the Chernobyl accident. Mutat. Res. 1997;381:267–278. doi: 10.1016/s0027-5107(97)00212-1. [DOI] [PubMed] [Google Scholar]
- 35.Kovalchuk O., Dubrova Y.E., Arkhipov A., Hohn B., Kovalchuk I. Wheat mutation rate after Chernobyl. Nature. 2000;407:583–584. doi: 10.1038/35036692. [DOI] [PubMed] [Google Scholar]
- 36.Ellegren H., Lindgren G., Primmer C.R., Moller A.P. Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature. 1997;389:593–596. doi: 10.1038/39303. [DOI] [PubMed] [Google Scholar]
- 37.Dubrova Y.E., Bersimbaev R.I., Djansugurova L.B., Tankimanova M.K., Mamyrbaeva Z.Z., Mustonen R., Lindholm C., Hulten M., Salomaa S. Nuclear weapons tests and human germline mutation rate. Science. 2002;295:1037–1037. doi: 10.1126/science.1068102. [DOI] [PubMed] [Google Scholar]
- 38.Bridges B.A. Radiation and germline mutation at repeat sequences: are we in the middle of a paradigm shift? Radiat. Res. 2001;156:631–641. doi: 10.1667/0033-7587(2001)156[0631:ragmar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Dubrova Y.E., Plumb M. Ionising radiation and mutation induction at mouse minisatellite loci: The story of two generations. Mutat. Res. 2002;499:143–150. doi: 10.1016/s0027-5107(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 40.Dubrova Y.E., Plumb M., Gutierrez B., Boulton E., Jeffreys A.J. Transgenerational mutation by radiation. Nature. 2000;405:37. doi: 10.1038/35011135. [DOI] [PubMed] [Google Scholar]
- 41.Wang B., Ohyama H., Nose T., Itsukaichi H., Nakajima T., Yukawa O., Odaka T., Tanaka K., Kojima E., Yamada T., Hayata I. Adaptive response in embryogenesis: I. Dose and timing of radiation for reduction of prenatal death and congenital malformation during the late period of organogenesis. Radiat. Res. 1998;150:120–122. [PubMed] [Google Scholar]