Abstract
Almost all of our knowledge about the mutational effect of radiation has come from high dose studies which are generally not relevant to public exposure. The pKZ1 mouse recombination mutagenesis assay enables study of the mutational effect of very low doses of low LET radiation (μGy to cGy range) in a whole animal model. The mutational end-point studied is chromosomal inversion which is a common mutation in cancer. We have observed 1) a non-linear dose response of induced inversions in pKZ1 mice exposed to a wide dose range of low LET radiation, 2) the ability of low priming doses to cause an adaptive response to subsequent higher test doses and 3) the effect of genetic susceptibility where animals that are heterozygous for the Ataxia Telangiectasia gene (Atm) exhibit different responses to low dose radiation compared to their normal litter-mates.
Keywords: Chromosomal inversion, X-radiation, Transgenic mouse mutagenesis model, Adaptive response
INTRODUCTION
It is known that as the dose of ionising radiation increases then the number of mutations resulting from that exposure increases. The relationship between dose of ionising radiation and DNA damage appears to be linear at high doses. The linear no threshold (LNT) model predicts that this relationship is also linear at low doses and that any dose above zero will result in some DNA damage. Almost all of the data on mutation in response to ionising radiation comes from studies of high doses. However, the population is normally unlikely to be exposed to high doses of radiation. There is little evidence that low doses of radiation (less than 0.1 Gy) are mutagenic and there is some suggestion that low doses of radiation may even be beneficial (Pollycove and Feinendegen 2003). It is important to determine whether there is a threshold dose at which no DNA damage occurs. There is, however, a paucity of in vivo or in vitro assays with the sensitivity to detect mutagenic responses to low doses of radiation. The majority of in vivo mouse studies showing the mutagenic effect of X-radiation have used doses > 1 Gy (Winegar et al. 1994; Gossen et al. 1995).
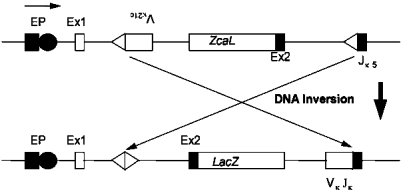
The pKZ1 transgenic recombination mutagenesis model has proven to be a sensitive model for detecting chromosomal inversions in spleen in response to very low doses of three known chemical carcinogens, cyclophosphamide (Sykes et al. 1998), etoposide (Hooker et al. 2002) and mitomycin C (unpublished results). In the case of cyclophosphamide the model has been shown to be 4 orders of magnitude more sensitive at detecting inversions than other mouse models are at detecting point mutations (Kohler et al. 1991; Sykes et al. 1998). The mice contain a transgene which has the E. coli β-galactosidase gene (lacZ) in reverse orientation to a chicken β-actin promoter complex. The lacZ gene is flanked by the mouse Vκ21C and Jκ5 immunoglobulin recombination signal sequences (Matsuoka et al. 1991). When an inversion event occurs, facilitated by the mouse recombination signal sequences, the lacZ gene is brought into correct juxtaposition with respect to the promoter complex and expression of the E. coli β-galactosidase message can occur (Fig 1). The E. coli β-galactosidase protein can then be detected in tissues in the animals by staining with the chromogenic substrate X-gal. Cells that undergo inversion events exhibit blue staining. The number of inversion events is determined by the ratio of the number of blue staining cells to the total number of cells counted. Inversion events have been detected in a wide range of pKZ1 tissues, with most studies to date concentrating on spleen tissue.
FIGURE 1.
The pKZ1 transgenic mouse construct. LacZ, E. coli, β-galactosidase gene; EP, chicken β-actin enhancer/promoter complex; Ex1 and Ex2, chicken β-actin exons 1 and 2; VK21C and JK5, mouse immunoglobulin recombination signal sequences.
We set out to determine if the pKZ1 recombination mutagenesis model would provide a sensitive mutation assay in response to single whole body doses of low dose, low LET radiation in order to determine if the LNT model applies at doses < 0.1 Gy. We also utilised the pKZ1 model to study adaptive response and genetic susceptibility in response to low dose radiation exposure.
METHODS
Mice
A colony of pKZ1 mice was maintained by breeding pKZ1 mice to C57/BL6J mice. Double transgenic mice were bred by crossing pKZ1 heterozygous mice with Atm heterozygous knock-out mice (Elson et al. 1994). Mice were screened for the presence of the lacZ transgene and the Atm knock-out construct in DNA from tail clippings using polymerase chain reactions specific for the E. coli lacZ gene and the Atm knock-out construct.
Irradiation of Mice
The mice were irradiated using a Philips deep X-ray unit. The machine was operated at 250 kV and 12 mA, HVL of 3 mm Cu, Source to Surface Distance (SSD) ranged from 80–291 cm and field size was 25 cm × 25 cm. The X-ray machine output was calibrated according to IPEMB protocol (Institution of Physics and Engineering in Medicine and Biology 1996). This dosimetry system has a calibration factor that is traceable to the Australian Radiation Protection and Nuclear Safety Agency.
Irradiation was carried out after placing mice in a perspex mouse holder. Radiation doses less than 0.01 Gy were delivered at a SSD of 291 cm and using additional lead filters to reduce the dose rate. The control mice were also placed in the perspex holder for the same period of time but without the irradiator turned on. After irradiation the mice were returned to their normal housing cages where they were housed for three days.
For adaptive response experiments, mice were exposed to a low priming dose of radiation and then a higher test dose 4 hours later. Control mice included sham-treated mice as well as priming dose only and test dose only.
Histochemical Detection of Inversion Events
The mice were euthanased by CO2 asphyxiation and dissected. The spleen was snap frozen in OCT cryoprotectant embedding compound (Tissue-Tek) and stored at −20ºC. Frozen tissue sections were cut on a cryostat and stained for β-galactosidase (β-gal) expression using the method described in Sykes et al (1998). Briefly, 5 μm frozen spleen sections were fixed in glutaraldehyde, stained with X-gal and counter-stained with neutral red. E. coli β-galactosidase expressing cells (ie blue staining cells) were counted using light microscopy. Sixty random fields within each spleen were screened. The number of blue staining cells/total number of cells screened, were recorded. pKZ1 transgenic mouse brain sections, which exhibit strong E. coli β-gal staining (Matsuoka et al. 1991), were used as a positive staining control. Spleen sections from a non-transgenic mouse were used as a negative control. All slides were coded by another individual in the laboratory and then the slides were scored blind. The data were analysed using a two-tailed Mann-Whitney U test.
RESULTS
Threshold
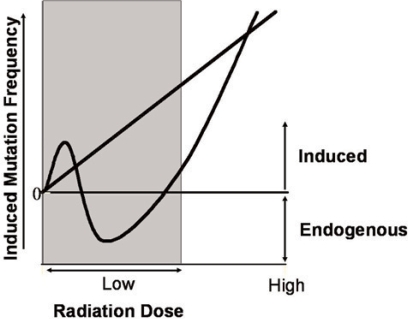
At high doses of radiation (> 0.1 Gy) an increase in chromosomal inversions was observed compared with the endogenous inversion frequency. Between 0.1 mGy and 100 mGy a decrease in inversions below endogenous frequency was observed, and at very low doses (< 0.01 mGy) an increase in inversions was again detected until the frequency returned to the endogenous frequency observed in the sham-treated animals.
Adaptive Response
Mice were treated with 0.01 Gy priming dose and then 1 Gy challenge dose 4 hours later. Control groups included sham-treated, priming alone and challenge alone. No difference was observed in mutation frequency between sham-treated animals and those receiving 0.01 Gy. There was a trend towards an increase in inversion frequency for the 1 Gy treatment groups. The groups that received a priming dose of 0.01 Gy followed by a challenge dose of 1 Gy 4 hours later showed a decrease in inversions to a level below endogenous frequency.
Genetic Susceptibility
pKZ1 mice which have two normal copies of the Atm gene and pKZ1 mice that were heterozygous knock-out for the Atm gene were exposed to 15 mGy of X-radiation. The sham-treated Atm heterozygous knock-out mice had an endogenous inversion frequency similar to the Atm normal pKZ1 mice. Irradiated Atm heterozygous knock-out mice did however demonstrate fewer inversions after exposure to 15 mGy X-radiation than the Atm normal pKZ1 mice.
DISCUSSION
The results presented here indicate that the pKZ1 model will be a useful whole animal model for studying the mutagenic effect of low dose radiation. We observed an inversion response after exposure to doses of radiation that are orders of magnitude lower than have previously been reported. The dose-response observed was non-linear at doses less than 0.1 Gy. At very low doses and at high doses an increase in inversions was observed, and at intermediate doses a decrease below endogenous inversion frequency was observed. The shape of the dose-response observed is shown in Figure 2. The only report of a whole animal model exhibiting an induction of mutations at X-radiation doses of < 0.1 Gy was by Scheistl et al. (1994) where they found a linear dose-response relationship between 1 cGy and 1 Gy. The mechanism behind the non-linear response observed here is not known. The responses seen at the lowest doses must involve the bystander effect as not all cells will sustain direct DNA damage from the low dose radiation. Inversions will result when stem and loop structures are resolved by non-homologous end-joining (NHEJ) repair. NHEJ will almost always result in a change to the DNA sequence and is therefore potentially mutagenic. An increase in inversions suggests an increase in NHEJ repair, and a decrease in inversions suggests a decrease in NHEJ repair. If double-strand DNA breaks are not repaired in a cell as a result of down-regulated NHEJ then the cell may apoptose, and therefore any mutations that have occurred in that cell will not become fixed. It is possible that the amount of DNA damage sustained at very low doses may not be enough to elicit a down-regulation of NHEJ. At high doses the cells may respond by up-regulating NHEJ in order to repair the very large number of DNA double-strand breaks that are sustained. The relevance of these results to human health will hinge on elucidating the down-stream effects of reduction and induction of inversion events at such low doses.
FIGURE 2.
Low dose radiation dose-response curve. Inversions were induced in pKZ1 spleen at very low and at high doses of radiation exposure. Intermediate doses of radiation caused a decrease below endogenous inversion frequency. The straight line represents the LNT theory.
The adaptive response can be defined as a change in sensitivity to radiation damage induced by a low dose, which is delivered prior to a larger challenge dose (Wolff 1998). This has the potential to affect the risk estimation of radiation exposure. The risk of damage from high dose radiation exposure may be decreased if there has been prior exposure to low dose radiation, affecting the validity of the risk predicted using the LNT theory. Our results strongly suggest that a low priming dose of 0.01 Gy is capable of reducing the frequency of inversions caused by a subsequent challenge dose of 1 Gy. The aim here was to determine if inversions could be used as an end-point to study adaptive response at doses of radiation that are lower than have previously been reported. These experiments suggest that the pKZ1 mouse mutation model is an appropriate whole animal system for measurement of the adaptive radiation response. The priming dose (0.01 Gy) used in these experiments is amongst the lowest priming dose reported to be capable of inducing an adaptive response in either in vivo or in vitro studies (reviewed in Wolff 1998). The decrease in mutation frequency that we observed in the experiments performed here is also amongst the largest decrease in mutation frequency below that induced by the challenge dose to have been reported.
Different individuals in the population will have different genetic susceptibilities to DNA damaging agents such as ionising radiation based on their ability to repair damaged DNA. Individuals homozygous for ATM mutations are sensitive to ionizing radiation. There is some evidence that individuals in the population who are carriers for an ATM mutation (approximately 1% of the population) may be more sensitive to radiation than normal individuals. Mouse Atm heterozygotes have an increased rate of cataract development after 0.5 – 4 Gy radiation exposure (Worgul et al. 2002) and decreased life span after exposure to 4 Gy (Barlow et al. 1999) compared with normal mice. In addition, lymphocytes from human ATM heterozygotes exhibit greater cell death in response to low dose-rate radiation than normal individuals (West 1995). We utilised the pKZ1 mouse model to determine whether carrying one defective Atm gene resulted in increased susceptibility to low dose radiation damage. Animals that were heterozygous for loss of function of an Atm gene exhibited a reduction in inversions below that of endogenous inversion frequency after a low dose radiation exposure compared with sham-treated heterozygous animals and also their normal litter-mates. The ATM protein plays an important role in both homologous recombination and NHEJ (Valerie and Povkirk 2003). A reduction in ATM protein in heterozygous animals may therefore lead to a reduction in NHEJ. Further study is required to determine the relevance of the reduction in NHEJ in ATM heterozygotes to human health.
DNA double strand breaks are known to be the major lesion induced after X-irradiation. The pKZ1 mutagenesis model allows measurement of inversion events, which arise through the repair of DNA double strand breaks. In spleen tissue in the pKZ1 model, inversion events may be recognized and repaired using the RAG1/RAG2 mediated NHEJ machinery which is important in both repair of DNA double strand breaks and in the normal development of Band T-cell receptors (reviewed in Lieber 1998). Investigation of the important enzymes in the NHEJ pathway (for example RAG-1, RAG-2, DNA-PK, Ku 70, Ku 86), will hopefully lead to further clues about the underlying mechanism of changes in inversions identified in the pKZ1 mouse spleen in response to low dose radiation.
The results presented here indicate that the pKZ1 mouse model has the sensitivity to enable study of low dose radiation exposure which is relevant to human population exposure. The model can also be used to study adaptive response and genetic susceptibility in response to low dose radiation exposure. Our data using DNA inversions as a mutagenic end-point indicate that the dose response to X-radiation is not linear and that the validity of the LNT model needs to be revisited. This will have an important impact when setting exposure limits and conditions for safe occupational and medical radiation exposure.
ACKNOWLEDGMENTS
We wish to thank Professor Martin Lavin (Queensland Institute of Medical Research) for kindly providing heterozygous Atm mice to establish the pKZ1/Atm mouse colony. Research funded by the Low Dose Radiation Research Program, Biological and Environmental Research (BER), U.S. Department of Energy, grant # DE-FG02-01ER63227.
REFERENCES
- Barlow C, Eckhaus MA, Schaffer AA, Wynshaw-Boris A. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nature Genet. 1999;21:359–360. doi: 10.1038/7684. [DOI] [PubMed] [Google Scholar]
- Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen JA, Martus HJ, Wei JY, Vijg J. Spontaneous and X-ray-induced deletion mutations in a LacZ plasmid-based transgenic mouse model. Mutat Res. 1995;331:89–97. doi: 10.1016/0027-5107(95)00055-n. [DOI] [PubMed] [Google Scholar]
- Hooker AM, Horne R, Morley AA, Sykes PJ. Dose-dependent increase or decrease of somatic intrachromosomal recombination produced by etoposide. Mutat Res. 2002;500:117–124. doi: 10.1016/s0027-5107(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Institution of Physics and Engineering in Medicine and Biology The IPEMB code of practice for the determination of absorbed dose for X-rays below 300 kV generating potential (0.035 mm A1 – 4 mm Cu HVL; 10–300 kV generating potential) Phys Med Biol. 1996;41:2605–2625. doi: 10.1088/0031-9155/41/12/002. [DOI] [PubMed] [Google Scholar]
- Kohler SW, Provost GS, Fieck A, Kretz PL, Bullock WO, Putman DL, Sorge JA, Short JM. Analysis of spontaneous and induced mutations in transgenic mice using a lambda ZAP/lacI shuttle vector. Environ Mol Mutagen. 1991;18:316–321. doi: 10.1002/em.2850180421. [DOI] [PubMed] [Google Scholar]
- Lieber MR, Warner-Lambert/Parke-Davis Award Lecture Pathological and physiological double-strand-breaks: roles in cancer, aging and the immune system. Am J Path. 1998;153:1323–1332. doi: 10.1016/s0002-9440(10)65716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Nagawa F, Okazaki K, Kingsbury L, Yoshida K, Muller U, Larue DT, Winer J, Sakano H. Detection of somatic DNA recombination in the transgenic mouse brain. Science. 1991;254:81–86. doi: 10.1126/science.1925563. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Khogali F, Carls N. Reversion of the mouse pink-eyed unstable mutation induced by low doses of X-rays. Science. 1994;266:1573–1576. doi: 10.1126/science.7985029. [DOI] [PubMed] [Google Scholar]
- Sykes PJ, Hooker AM, Harrington CS, Jacobs AK, Kingsbury L, Morley AA. Induction of somatic intrachromosomal inversion events by cyclophosphamide in a transgenic mouse model. Mutat Res. 1998;397:209–219. doi: 10.1016/s0027-5107(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Valerie K, Povkirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- West CM, Elyan SA, Berry P, Cowan R, Scott D. A comparison of the radiosensitivity of lymphocytes from normal donors, cancer patients and individuals with ataxia telangiectasia (A-T) and A-T heterozygotes. Int J Radiat Biol. 1995;68:197–203. doi: 10.1080/09553009514551101. [DOI] [PubMed] [Google Scholar]
- Winegar RA, Lutze LH, Hamer JD, O'Loughlin KG, Mirsalis JC. Radiation-induced point mutations, deletions and micronuclei in lacI transgenic mice. Mutat Res. 1994;307:479–487. doi: 10.1016/0027-5107(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Wolff S. Aspects of the adaptive response to very low doses of radiation and other agents. Mutat Res. 1996;358:135–142. doi: 10.1016/s0027-5107(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106(Suppl. 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgul BV, Smilenov L, Brenner DJ, Junk A, Zhou W, Hall EJ. Atm heterozygous mice are more sensitive to radiation-induced cataracts than are their wild-type counterparts. Proc Natl Acad Sci USA. 2002;99:9836–9839. doi: 10.1073/pnas.162349699. [DOI] [PMC free article] [PubMed] [Google Scholar]