Abstract
Three aspects of hormesis with low doses of ionizing radiation are presented: the good, the bad, and the ugly. The good is acceptance by France, Japan, and China of the thousands of studies showing stimulation and/or benefit, with no harm, from low dose irradiation. This includes thousands of people who live in good health with high background radiation. The bad is the nonacceptance of radiation hormesis by the U. S. and most other governments; their linear no threshold (LNT) concept promulgates fear of all radiation and produces laws which have no basis in mammalian physiology. The LNT concept leads to poor health, unreasonable medicine and oppressed industries. The ugly is decades of deception by medical and radiation committees which refuse to consider valid evidence of radiation hormesis in cancer, other diseases, and health. Specific examples are provided for the good, the bad, and the ugly in radiation hormesis.
Keywords: cancer, health, therapy, radon, BEIR VI, deception
INTRODUCTION
Hormesis is the stimulation of any system by low doses of any agent (Luckey, 1980a). Large and small doses of most agents elicit opposite responses. A dose that elicits a response which separates positive from negative effects is the threshold dose; it is the “zero equivalent point” (ZEP) for that specific parameter. Low dose is any dose below ZEP. Dose rate is also important. Taking one pill per day may be life-saving; taking 365 of most pills in one day would be lethal.
Radiation hormesis is the stimulation, often considered to be beneficial, from low doses of ionizing radiation. Large doses are harmful. The difference is quite clear in those dose-response curves which involve both biopositive and bionegative effects. At any given rate, the physiologic response to ionizing radiation is directly proportional to the logarithm of the dose (Luckey, 1991).
Following William Crookes' inventions of the radiometer in 1875 and the cathode ray tube in 1877, physicists began to understand the dualistic nature of radiation. Fundamental discoveries made in the last decade of the 19th century (Table 1) are the foundation for the use of ionizing radiation in physiology, immunology, medical diagnosis, and therapy (Brucer, 1990). Ionizing radiation includes electromagnetic rays and high linear energy particles. The rays are high energy photons: ultraviolet (UV) rays, X rays, and gamma rays. Ionizing particles include alpha rays (nuclei of the element, helium), beta rays (electrons), protons (hydrogen with only one electron), and neutrons. Each ray and particle has a wide range of energies (often expressed as million electron volts (MeV)) which is defined by its source. The physiologic effects of other rays and particles (neutrinos, muons, positrons, and atoms) are poorly defined.
TABLE 1.
Nineteenth century tools for ionizing radiation (Brucer, 1990)
| Year | Item | Person and Action |
|---|---|---|
| 1890 | Isotope | P. Schutzenburger suggested there are isotopes for many elements. |
| 1891 | Electron | G. Stoney defined the electron as one unit of electric charge. |
| 1892 | U Radiation | W. Crookes found that uranium fogged photographic film. |
| 1893 | Hydrogen | J. Mallet suggested hydrogen should be the unit atomic weight. |
| (Proton | In 1920, E. Rutherford called the hydrogen atom a proton.) | |
| 1894 | X Ray | A. Goodspeed threw away “freak” pictures found on some film. |
| 1895 | X Ray | W. Roentgen found (Nov. 8) Crookes tube rays pass through cardboard. |
| J. Thomson found X rays ionize air. | ||
| Helium | Ramsay & Rayleigh discovered helium in the gas from uranium ore. | |
| Ion Paths | C. Wilson built the first cloud chamber. | |
| 1896 | X Ray | The X ray of Mrs. Roentgen's hand appeared in January news papers. |
| Diagnostic X rays began in many countries. | ||
| T. Edison invented the fluoroscope. | ||
| W. Shrader found high doses of X rays kill bacteria. | ||
| Many reported dermatitis from X rays. | ||
| H. Becquerel discovered uranium produced this new radiation. | ||
| Therapy | H. Gocht treated cancer; L. Freund treated inflammation. | |
| Hormesis | Schrader and Loret showed low doses of X rays stimulated immunity. | |
| New York Health Dept. found low doses stimulate TB bacilli. | ||
| E.M. Rays | E. Rutherford detected electromagnetic waves. | |
| 1897 | Electron | J. Thomson discovered electrons. |
| Gamma Ray | J. Thomson discovered gamma rays. | |
| Sickness | D. Walsh described radiation sickness. | |
| Therapy | A. Ausset reported X rays helped a moribund patient with tuberculosis. | |
| RPC | Radiation Protection Committee formed in England. | |
| 1898 | Actinium | A. Debierne discovered actinium produced rays. |
| Radium | M. Curie discovered radioactive radium. | |
| Polonium | M. Curie discovered radioactive polonium. | |
| Radioactivity | M. Curie found thorium and potassium are radioactive. | |
| 1899 | Radon | E. Rutherford noted thorium produces a gas (radon). |
| Half-life | E. Rutherford used decay rates of radioactive elements for half-life values. | |
| Alpha Ray | E. Rutherford discovered alpha rays. | |
| Beta Ray | E. Rutherford discovered beta rays. | |
| S. Meyer noted that beta rays are electrons. | ||
| Gamma Ray | E. Rutherford defined gamma rays. | |
| Dermatitis | M. Curie found X rays caused skin erythema. | |
| Neutron | S. Sutherland named neutrons. | |
| Actinium | A. Debierne discovered actinium and its radiation. | |
| Hormesis | J. Loeb found radiation stimulated parthenogenesis in sea urchins. |
Although all elements decay, only tritium, carbon, potassium, and those heavier than bismuth are usually considered to be naturally radioactive. Radioactive atoms are used in diagnostic and therapeutic medicine, and in industry; some occur naturally and some are man made. Natural radiation is in the air, water, earth, our bodies, and all the materials we use. In general, adults receive about 2 mGy/y, about 10,000 radiations per minute, from natural sources. Brucer estimated that 25% of all medical patients received some radiation, the average is about 0.54 mGy/y (Brucer, 1990).
Each minute some of the ten trillion cells in our body receive ionizing radiation from our natural background (Luckey, 1991). Depending upon its energy, one alpha ray might hit 2–50 cells (the 7 MeV alpha rays of radon progeny traverse 0.07 mm tissue), a beta ray could hit 10–500 cells (a 1 MeV beta ray penetrates 6.4 mm tissue), and each X and gamma ray might hit 1,000–100,000 cells.
The initial action of most ionizing radiation is on the water which constitutes about 98% of the total number of molecules in soft tissues. Ionizing radiation produces a variety of oxygen species from water (Table 2) (Gould, 1968, Luckey, 2005a). Each of these will avidly attack nearby material to make strange compounds and atomic fragments (free radicals) which can change the structure of DNA and RNA, drastically alter metabolic pathways, and kill bacteria and tissue cells. Low dose irradiation is insufficient to cause erythema or to kill healthy mammalian cells. If the rate of destruction is not too fast, damage in healthy tissues can be bypassed or repaired and the overall reaction is biopositive (Polycove and Feinendegen, 2003). A major effect is activation of the immune system. There is also a powerful direct action upon anaerobic bacteria which cause infection, gangrene for example. Each oxygen specie is devastating for anaerobic bacteria. Necrosis stops when the bacterial toxins are neutralized and our macrophages start the cleaning and healing processes.
TABLE 2.
Oxygen species in irradiated tissues (Gould, 1968)
| Species | Name |
|---|---|
| H3O+ | Hydronium ion |
| H2O+ | Oxonium ion |
| HO+ | Hydroxonium ion |
| HO· | Hydroxyl radical |
| HO·2 | Perhydroxyl radical |
| O· | Oxygen radical |
| O: | Atomic oxygen |
| O2 | Molecular oxygen |
| O3 | Ozone |
| O2− | Superoxide ion |
| O22− | Peroxide ion |
| O3− | Ozonide ion |
| HO2− | Perhydroxide ion |
| H2O2 | Hydrogen peroxide |
A broad view of radiation hormesis includes the good, the bad, and the ugly. The good includes abundant evidence showing increased 1) physiologic performance, 2) immune competence, 3) health, and 4) mean lifespan. Some evidence indicates ionizing radiation is essential for life (Luckey, 2004). The bad is the false concepts that “all radiation is harmful” and partial dose-response curves that are “linear with no threshold” (LNT). This leads to fear of low doses of ionizing radiation. The bad also includes the cessation of beneficial uses of ionizing radiation without reason. The ugly is the consistent misrepresentation of biopositive effects by established scientists and our government advisory committees. With no direct evidence of harm, they ignore overwhelming evidence showing benefits from low dose irradiation. Examples of the good, the bad, and the ugly are provided.
THE GOOD
Over 3,000 scientific research papers show that low dose irradiation is stimulatory and/or beneficial in a wide variety of microbes, plants, invertebrates, and vertebrates (Luckey, 1980a, 1991, Muckerheide, 2001). Using the parameters of cancer mortality rates or mean lifespan in humans, no scientifically acceptable study was found which showed that less than 10 cGy was harmful. Radiation, Science, and Health, Inc. (Box 843, Needham, MA 02494) offers $1,000 for one report in English with scientifically acceptable evidence of harm (increased cancer death rate or decreased average lifespan) from low dose irradiation in normal (not immune deficient) humans or laboratory animals. This is opposed by several thousand studies which produced confirmed and definitive evidence of stimulation and/or benefit.
The good is acceptance of radiation hormesis by the French Academy of Sciences (Aurengo et al., 2005). That thorough and exemplary document makes it very clear that the Linear No Threshold (LNT) concept is not tenable. “However, the use of LNT in the low dose or low dose range is not consistent with the current radiobiological knowledge.” (p. 10) For doses <10 cSv, epidemiologic studies “have not been able to detect statistically significant risk even on large cohorts or populations.” (p.8) “Indeed, a meta-analysis of experimental data shows that in 40% of animal experiments there is a decrease in the incidence of spontaneous cancers after low doses.” (p.9) “These data show that the use of a linear no-threshold relationship is not justified for assessing by extrapolation the risk of low doses…” (p. 9) “In conclusion, this report doubts the validity of using LNT in the evaluation of the carcinogenic risk of low doses (<100 mSv) and even more for very low doses (<10 mSv).” (p.10) “For doses lower than 100 mSv, almost all studies do not evidence a significant effect.” (p. 25) Regarding leukemia in atomic bomb victims: “the dose-effect relationship is statistically incompatible with an LNT relationship,” (p. 25) “The LNT model cannot be used to estimate the effect of very low doses…” (p36)
The good includes the natural occurrence of increased ionizing radiation in certain parts of the world. Areas in Brazil, Egypt, Iran, and India have up to 20 times more radiation than the US average of 2 mGy/y (Cullan and Franca, 1977). Brazilians flock to beaches which have high radiation levels, 0.03 mGy/h, from black monazite sands. Many health spas throughout the world contain radioactivity comparable with the above sites; much of this is due to radium and radon in the water.
In a 600 page report, the French Academy of Sciences document other groups subjected to unusual levels of ionizing radiation with either no or beneficial effects (Aurengo et al., 2005). 1) The incidence of solid cancers decreased in 21,500 exposed workers at Mayak, a Russian plutonium production complex. 2) The total cancer deaths in 8,600 cleanup workers at Chernobyl (who received an average of 5 cGy) was 12% lower than that of the general Russian population. 3) The leukemia death rate in 96,000 nuclear workers (in three countries) exposed to over 40 cSv was only half that predicted. 4) No increased cancer was found in 222,400 radiologists and radiation technicians who received more than 20 cGy in 20 years. 5) There was no increased cancer rate in 46,740 flight crews (mostly European) who received over 1.5 mGy/y. 6) Repeated diagnostic exposures of patients who have received less than 10 cGy radiation results in no perceptible leukemia, the most radiosensitive of all cancers. 7) There was no increased cancer in adjoining tissues which received less than 5 cGy radiation in 160,000 women exposed to high doses of radiation to the cervix. 8) No excess thyroid cancer was found in two million children who were irradiated by the Chernobyl explosion. 9) Twin pregnancies receive twice the number of diagnostic radiobiologic examinations as do single pregnancies; some studies show considerably reduced cancer incidence in twins.
The best of the good is the work of Dr. Sadao Hattori (Fig. 1). He recognized the implications of radiation hormesis: “If radiation hormesis exists, our daily activities in radiation management have been extremely erroneous.” (Hattori, 1994). Following a thorough literature review, the Central Research Institute of the Electric Power Industries (CRIEPI) inaugurated 15 research projects at 10 Japan universities. The resulting research papers, published in peer reviewed journals, confirmed the radiation hormesis thesis: low dose irradiation stimulates many physiologic parameters that are consistent with damage control and improved health. Today, parts of both government and industries of Japan accepted the concept of radiation hormesis. Some health care centers and hospitals in Japan use low dose radiation therapy (Sakamoto and Myojin, 1996).
FIGURE 1.

Dr. Sadao Hattori, retired Senior Vice President and Director of Research, Central Research Institute of the Electric Power Industries (CRIEPI), Tokyo. (With permission of Dr. Hattori)
CANCER
The best of the good includes the pioneering research of Dr. K. Sakamoto (Fig. 2) and associates who showed that low dose irradiation of the torso was the most effective treatment for malignant lymphoma (Sakamoto, 1996, 1997). Exposure of either the head and neck or the lower half of the body were without effect. They had previously established this selective area for low dose irradiation using decreased cancer death rates in mice. Sakamoto's concept was confirmed by a survey of 14,137 lymphoma patients treated with low dose, total body irradiation. “Data indicate that half of the patients in stage I (indolent lymphoma) are cured (with a 15 year follow-up) by radiotherapy alone. Addition of chemotherapy to radiotherapy does not indicate any improvement in overall outcome.” (Gustavsson et al., 2003).
FIGURE 2.

Dr. Kiyohiko Sakamoto, retired Professor, Medical School, Tohoku University, Sendai. (With permission of Dr. Sakamoto)
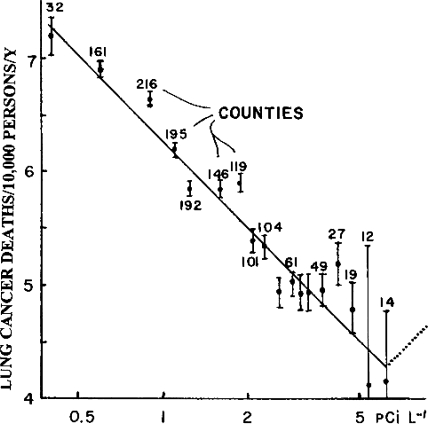
The best of the good includes the epic study of Cohen which showed that lung cancer deaths decreased with increased radon concentration in homes (Fig. 3) (Cohen, 1995). The increased variability near 5 pCi/l suggests that 8 pCi/l is about the optimum level for radon in homes. Radon concentration was the only one of 54 epidemiologic parameters which showed good correlation between increased radon concentration and decreased lung cancer death rates. Other studies confirm this benefit from low doses of radon (Bogoljubov, 1988, Becker, 1995, 2005, Deetjen, 1998). One must conclude that increased home radon reduces lung cancer.
FIGURE 3.
Home radon decreases lung cancer deaths. Data of B. Cohen (1995) from 200,000 radon samples from 1,600 counties in which live 90% of the United States population. One standard deviation is displayed. The dotted line suggests the optimum radon level is about 8 pCi/l.
A serendipitous good appeared in Taiwan (Chen et al., 2004). In 1983, about 10,000 Taipei residents moved into new apartments built with cobalt-60 contaminated steel bars. For two decades residents received an average of 1.5 cGy/y (the range was 0.1 to16 cGy/y), about 10 times more radiation than ambient levels in Taipei. In the first decade the cancer mortality rate of this exposed population dropped from 50 to 4 per 100,000 people while that of general population increased from 82 to 108 per 100,000. In the second decade the cancer death rate of the exposed residents remained at 3 per 100,000 people and that of the aging general population rose to 153 per 100,000. The Taiwan experience confirms reports from China.
A decades long epidemiologic study in China showed peasants living with three times the levels of natural radiation are more healthy in almost every characteristic than peasants living with lower levels of radiation (Luckey, 1991). The immunologic research of Liu and associated in Changchung provided understanding for some of the effects of low dose irradiation (Liu, 2003). China's current nuclear power program, with 10 nuclear power plants under construction and 100 more planned, indicates China rejects fear from low dose irradiation (Aurengo et al., 2005).
REPRODUCTION
When compared with non-irradiated controls, cohorts exposed to low dose irradiation show statistically significant increased physiologic functions (Luckey, 1991). Lightly irradiated rodents were more fertile than controls through several generations (increased ovulation in dams, increased number, viability and growth rates of young, and faster physical development of young) with no evidence of mutations in the young exposed in utero. Irradiated colonies were maintained in good health through 21 generations.
Kaplan (Kaplan, 1949) successfully treated sterility in women with low dose irradiation (about 90 cGy of X rays to the ovary in three weeks). Three generations showed no adverse effects. There was no evidence of genetic damage from 351 pregnancies in 644 irradiated women, “… the incidence of genetic damage to the children and grandchildren of this group is less than that in the normal population.”
IMMUNITY
Low dose irradiation activates the immune system in several ways: faster wound healing, and increased resistance to toxins, infections, and tumor cell injections (Luckey, 1991). Lightly irradiated animals survived doses of radiation which killed all unexposed controls. Lymphocyte production was increased by low dose irradiation. The search and destroy function of lymphocytes is facilitated by destruction of the radiation sensitive T repressor cells; this allows other T cells to be more efficient (Hellstrom and Hellstrom, 1979). When compared with strict controls, cancer mortality rates were significantly decreased (almost 50%) in accidentally irradiated nuclear workers (Luckey, 1991, 1997a).
The good certainly includes Dr. Liu and Dr. Ina and their associates who have revealed metabolic details of immune activation by low dose irradiation (Liu, 2003, Ina and Sakai, 2004, 2005). Changes in cell functions and enzyme characteristics support the radiation hormesis thesis. Cell concentrations of many important components of the immune system (enzymes and metabolites) are increased by low dose irradiation of the host.
For the first four decades of the last century, low dose irradiation (“mild radiation therapy”) was the recommended treatment for many human disease states; it was particularly effective for infections from anaerobic bacteria (Kelly and Dowell, 1942, Berk and Hodes, 1991). Low dose irradiation was recommended for the non-invasive treatment of gas gangrene and severe ulcerative gingivitis (Luckey, 2005a, 2005b).
Low doses of radon are also effective. Good scientific data came from thousands of patients at two large radon hospitals in Russia (Bogoljubov, 1988). In this study, the placebo gas was additional nitrogen. More scientific data were provided by Deetjen with double blind studies comparing radon water and pure water baths in patients with a variety of illnesses (Deetjen, 1998). Every year, thousands of people visit radon rich mines to alleviate a wide variety of diseases and pains with and without the concurrence of the medical profession (Salak, 2003, Becker, 2004, Lewis, 2005). Anecdotes about improved health and relieved pain by these patients are substantially ignored. Increased immune competence contributes to increased mean lifespan in lightly irradiated laboratory animals and humans.
LIFESPAN
Low dose irradiation produced statistically significant increased average lifespan of laboratory animals and humans (Luckey, 1991, Ina and Sakai, 2004). Japanese bomb survivors exposed to low dose irradiation have statistically significantly longer average lifespan than those of control populations (Mine, 1991). When compared with the control population, the risk of non-cancer deaths in 22,777 Japanese atom bomb survivors increased only when the dose exceeded 155 cGy (Shimizu, et al. 1992, Pierce and Preston, 2001).
RADIATION IS AN ESSENTIAL AGENT
The ultimate good is: ionizing radiation is essential for life. All the criteria of an essential agent are met by low dose irradiation (Luckey, 1991, 2004).
When ionizing radiation is lowered below ambient levels, a wide variety of animals either do not survive, or become weak and perform poorly (Luckey, 1991, 1999a, Ruda and Kuzin, 1991). This is evidence that a radiation deficiency developed. Ionizing radiation uniquely prevents these syndromes. Low level irradiation increased the growth (replication) rate in protozoa (Luckey, 1991). Ionizing radiation promoted photosynthesis in both the presence and absence of light (Conter, et al. 1983, Luckey, 1980b). This suggests that radiation is a major source of energy for the abundant life at deep sea fissures and microbial metabolism underground in the deep hot biosphere (Gold, 1998). Supplementation with low dose irradiation lowered the cancer death rate, reduced infectious diseases, and provided a longer, healthy life in humans (Luckey, 1997b).
Premature cancer deaths are caused by insufficient radiation. Good evidence comes from 151,676 accidentally exposed workers in the nuclear industry (nuclear ships, bombs, and power plants) (Luckey, 1997a, 1999a, 1999b). There was no “healthy worker effect” because the exposed workers were matched with unexposed workers in equivalent jobs in the same factory with the same sex, age, economic, and sociologic conditions. When the average was weighted according to the number of participants in each study, the results showed that irradiation decreased the total cancer death rate 48%. These data indicate the United States has about 275,000 preventable, premature cancer deaths each year. The cause is attributed to insufficient radiation.
Each of the above examples indicates that we live in a state of partial radiation deficiency. The combined effects suggest we need radiation supplementation for more abundant health (Luckey, 1997b).
RADIATION THERAPY
Historically, medical uses of ionizing radiation have received deserved enthusiasm. The January 1896 publication of the X ray picture of Mrs. Roentgen's hand in the London Times made it “the most famous photo in history”. This quickly led to the use of X rays for diagnosis and 1000 papers were published within one year (Brucer, 1990). Physicians and radiation physicists soon learned that an excess of X rays produced erythema, skin lesions, and cancer. Diagnostic radiation methods continued to be refined throughout the 20th century. Many patients walk out of hospitals carrying enough radioactivity from diagnostic compounds to cause them to be rejected if they tried to enter a nuclear reactor facility with portal monitors (Brucer, 1990).
Both large and small doses of X rays were used therapeutically (Brucer, 1990). In 1897, H. Gocht treated breast cancer, E. Ausset treated tuberculosis, and L. Freund treated inflammation with X rays. In 1901, G. Phaler reported remarkable success in treating skin cancer with X rays. In 1902, therapy with low dose irradiation became a popular medical treatment for cancer and infections. Low doses were generally used: “…doses approaching the SED (skin erythema dose) are less successful than those treatments following the lower dose.” (Borak, 1944). Responses were generally very positive, prompt, and often relieved pain with no side effects. Numerous reviews indicate the value of low dose radiation therapy for skin eruptions, eye infections, pneumonia, and gangrene (Cuttler, 2002, Heidenhain, 1926, Desjardins, 1931, 1942, Kelly and Dowell, 1942, Berk, 1991, Calabrese and Baldwin, 1999, Luckey, 2005a). Low dose irradiation became popular and effective therapy for some cancers. Brucer noted “Between 1910–1950 hundreds of thousands of patients (no one knows how many) received millicuries (no one knows how much) of radium.” (Brucer, 1990). Radium and radon were successfully administered in tubes, needles, seeds, ointments and injection. “Mild radiation therapy”, as distinguished from high dose irradiation, was fashionable in Europe and promoted in Marie Curie's 1921 lecture tour of America (Macklin, 1993). Radium containing harnesses were designed to activate the thyroid (necklace), adrenal glands, ovaries (waist), and genitals (jockstrap). When he was dying with cancer, I helped Petr Beckman with a harness to fit a uranium rock close to his spleen.
The successes of radiation therapy for infection and inflammatory diseases abruptly ended in 1948. The financial advantage of antibiotics, the miracle drugs of World War II, made obsolete therapy with low dose irradiation. This was not good.
THE BAD
The bad is the promulgation of a false concept by many radiobiologists: all radiation is harmful. Brucer noted Health Physics had become a religious cult: “In 1979 the National Committee for Radiation Protection (NCRP) … dropped all pretense at science and assumed there was a risk in every radiation exposure.” (Brucer, 1990). In their attempt to obtain research money, geneticists predicted that genetic monsters (found in fruit flies subjected to large doses of radiation) would occur in people exposed to radiation from atom bombs. When geneticists chanted “all radiation is harmful” and “genetic monsters”, financial support for research on the effects of low dose irradiation vanished. Suddenly, editors were not interested in papers showing stimulatory or beneficial effects from low doses of ionizing radiation. Although no genetic monsters can be attributed to low dose irradiation, including atomic bombs, laws are based upon the false dogma that all radiation is harmful. “This is the greatest hoax of the twentieth century.” (Jaworowski, 1994).
The bad was the retreat to 19th century therapy, surgery, for patients with gangrene when bacteria became resistant to antibiotics about 30 years ago. Surgery is traumatic and the death rate from gangrene in diabetics remains high. “We have found only one really efficient means of prevention and treatment (of gas gangrene), and that is X-ray therapy without amputation, chemotherapy, or serum.” (Kelly and Dowell, 1942). Kelly and Dowell summarized 97 case histories of patients with gas gangrene and other infections: “One cannot fail to observe …. favorable changes in the clinical signs if one treats a few patients with such diseases after they appear to be too seriously ill to be moved from bed for any purpose.” Their conclusion was that neither chemotherapy nor serum was comparable with, nor compatible with, X ray treatment.
The bad is fear of low levels of radiation. The Nuclear Energy Agency (NEA) provided examples which include the hysteria in Europe from the radioactive cloud from Chernobyl (NEA, 1995). Thousands of needless abortions and suicides could have been prevented by education about low doses of radiation. Another example is the quandary people and governments of eastern Europe have about soils containing increased radiation from bombs containing spent uranium; they can not export their produce which has a small increase in radioactivity (Jovanovic, 2005). If they knew the facts, they would welcome increased low levels of radiation. Fear of radiation would cause devastating disruption and evacuation of cities following a terrorist attack with a dirty bomb. Neither officials nor the media understand that education about low dose irradiation is a defense against this form of terror. Excepting those who feel the blast, or who receive physical harm from heat or flying debris, low dose irradiation is beneficial (Luckey, 2004).
The bad is the denial of health by laws which restrict radiation in homes and industry to near ambient levels. The bad is the billions of dollars wasted by United States industry following the Environmental Protection Agency (EPA) rulings which were recommended by the National Committee for Radiation Protection (NCRP) and the Biological Effects of Ionizing Radiation (BEIR) committees; these were based upon the dogma that all radiation is harmful (Muckerheide and Rockwell, 1997). “As soon as Health Physicists saw the money in radiation hysteria, the Maximum Permissible Dose came down to background.” (Brucer, 1990).
A major bad is banning radioactive drugs by the U. S. Food and Drug Administration (FDA) without due process. This termination was triggered by the press coverage of a gruesome facial cancer and death of a celebrated millionaire, Eben Byers, who overdosed with 1500 vials of Radithor (each had 1 uCi Ra-228 and 1 uCi Ra-226 in 1/2 ounce of water) from 1928 to 1931 (Macklin, 1993). FDA ignored the benefits to thousands of people who took recommended doses.
The bad is: BEIR IV and BEIR VI committees downplayed the epic research of Cohen which proved (p < 0.00001) that home radon reduces lung cancer death rates (Cohen, 1995). They rejected radiation hormesis without providing any studies showing harm from ambient levels of radon. “…in the absence of credible evidence to the contrary, the committee adopted a linear-nonthreshold model for the relationship between radon exposure and lung cancer risk.” (BEIR VI, 1999, p. 6, emphasis added). Cohen's data (Fig. 3) show the EPA recommendation to reduce home radon levels to less than 4 pCi/l is carcinogenic (Luckey, 1993).
The voluminous BEIR VII provides no solid dose-response data to prove or refute the committee statements (BEIR VII, 2005). The abandonment of scientific principles by national and international radiation protection committees is so bad, it is ugly. A few examples of many deceptions by radiobiologists are provided (Luckey, 2000).
THE UGLY
The ugly is the misguided conclusion about low dose radiation by authorities who advise the U. S. government. These include the BEIR and NCRP committees, and their international counterpart organizations, the ICRP and UNSCEAR. BEIR committee members are appointed by a permanent group within the National Research Council, previously the Board of Radiation Effects Research (BRER), recently changed to the Nuclear and Radiation Studies Board (NRSB). The conclusions of these committees are not consistent with the results of the studies they report; nor do they consider confirmed results of thousands of studies that show hormesis and beneficial effects. The BEIR committees are drawn from, and supported by, the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine; “…the Academy has a mandate that requires it to advise the federal government on scientific and technical matters.” (BEIR, 1999, p. vi). These committees have not substantively considered the voluminouss evidence showing low dose irradiation is stimulatory and/or beneficial. Luckey listed 55 reviews published between 1907 and 1977 showing low dose irradiation was stimulatory (Luckey, 1980b). There are now over 3,000 peer reviewed research papers showing biopositive effects of low dose irradiation (Luckey, 1980a, 1991, Muckerheide, 2001).
RADON AND LUNG CANCER DEATH IN MINERS
The BEIR committees have 1,100 pages devoted to the false dogma that radon and its progeny cause lung cancer in humans (BEIR, 1988, 1999). They offer no creditable scientific evidence that radon increases lung cancer mortality in humans.
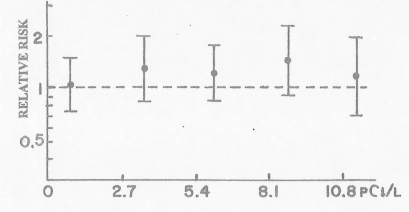
In order to evaluate the effect of radon in lung cancer deaths in homes, BEIR committees relied heavily on data from miners. “However, the results of dose models were used to extrapolate lung-cancer risks derived from the epidemiological studies of underground miners to the general population in indoor environments.” (BEIR, 1988) “…the BEIR VI committee chose to use the lung cancer information from studies of miners, who are more heavily exposed to radon, to estimate the risks posed by radon exposures in homes.” (BEIR, 1999). Their base was 11 major studies involving 68,000 miners and 2,700 lung cancer deaths. Their summary data (Fig. 4) showed conclusively that radon does not cause lung cancer deaths in miners (BEIR, 1999). Obviously, carcinogenic particulates and/or noxious gases, not radon and its progeny, must cause lung cancer in miners.
FIGURE 4.
Lung cancer deaths from radon in miners. Pooled analysis of the relative risks of lung cancer deaths, with 95% confidence limits, from 11 underground miner studies (adapted from p. 89, BEIR VI, 1999).
The BEIR VI committee continuously misrepresented their own data on lung cancer deaths in miners (BEIR, 1999). Compare their data (Fig. 4) with their statements:
p. 2 “The committee agreed with several earlier groups of experts that the risk of developing lung cancer increases linearly as the exposure increases; for example, doubling the exposure doubles the risk, and halving the exposure halves the risk. Furthermore, the existing biologic evidence suggests that any exposure, even very low, to radon might pose some risk.” (emphasis added)
p. 4 “ Radon, a naturally occurring gas formed from the decay of uranium in the earth, has been conclusively shown in epidemiologic studies of underground miners to cause lung cancer.” (emphasis added)
p. 18 “The carcinogenicity of radon is convincingly documented through epidemiologic studies of underground miners, all showing a markedly increased risk of lung cancer.” (emphasis added)
p. 114 “In each of these studies, miners have been shown to be at excess risk for lung cancer under past conditions of exposure.”
These repeated statements by the BEIR VI committee are contradicted by their own data. The BEIR VI dose response curves (Figures 4 and 5) show that radon and its progeny do not cause lung cancer deaths in homes or mines.
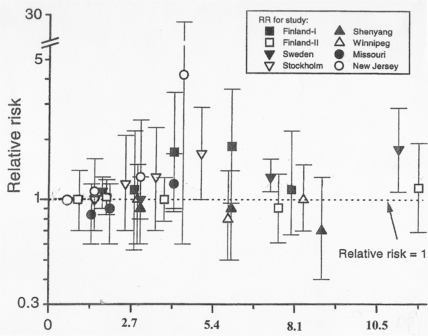
FIGURE 5.
Lung cancer deaths from radon in homes. Eight case control studies of relative risks for lung cancer death rates of people in homes (adapted from p. 377, BEIR VI, 1999). Units for the abscissa are pCi/l. One standard deviation is indicated.
RADON AND LUNG CANCER DEATH IN HOMES
The BEIR VI committee provides sparse data on lung cancer deaths in homes from case-control studies (BEIR, 1999). Eight studies with 4,263 lung cancer cases and 6,612 controls were examined using complex “meta analysis”. Their summary data (Fig. 5) show that the relative risk of lung cancer deaths did not rise significantly with increased radon concentrations. The committee conclusions are not consistent with their own data (Fig. 5) as shown in the following statements:
p. 9 “The committee selected a linear-nonthreshold relationship relating exposure to risk for the relatively low exposures at issue for indoor radon.”
p. 19 “Nonetheless, this indicates a public-health problem and makes indoor radon the second leading cause of lung cancer after cigarette-smoking,…”
p. 19 “Perhaps one-third of the radon-attributed cases (about 4% of the total lung-cancer deaths) would be avoided if all homes had concentrations below the Environmental Protection Agency's action guideline of 150 Bqm−3 (4 pCiL−1);”
p. 356 “… the data do support a small increase in lung cancer risk due to indoor radon…”
These misleading statements are then promulgated by the media, and used as advertising for a new industry, to take advantage of the misinformed public: lower the radon in your homes (BEIR, 1988). The BEIR VI committee denigrates Cohen's definitive study (Fig. 3) which proves that higher residential radon levels consistently decrease the lung cancer mortality. These data show that lowering radon in homes, as recommended by the EPA, will cause many lung cancer deaths.
RADIATION VS CANCER DEATHS IN OTHER COUNTRIES
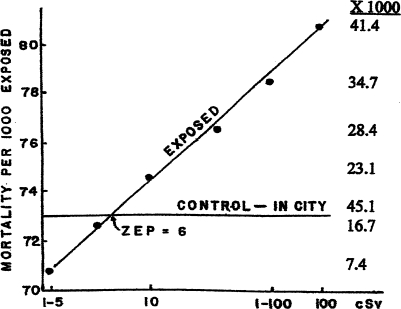
Ugly taints much of the good work done by the Radiation Effects Research Foundation (RERF), originally the Atomic Bomb Casualty Commission (ABCC), in Hiroshima which received millions of dollars each year for 50 years to study the effects of ionizing radiation on victims of atom bombs. The threshold appears to be 6 cSv. To their benefit, RERF authors did note that the cancer death rate of the control group (>3 km from the epicenter), which received low dose irradiation (0.2–0.6 cSv), was lower than that for persons not in the city at the time of the bombing (the original control group) who received less ionizing radiation (Shimizu et al., 1992). This result shows hormesis.
Over 23,000 Japanese atom bomb victims received less than10 cGy; their cancer death rates were no greater than that of the 34,272 persons in the control population. A graph of cumulative total cancer death rates in Japanese atom bomb victims illustrates that small and large doses of ionizing radiation elicit diametrically opposite results (Fig. 6) (Shimizu, 1992, Luckey, 1991). The RERF conclusion is misleading: “In general, the dose response … failed to suggest the existence of hormesis.” (Shimizu, 1992, p. 74). Since the chi square statistic showed the group with the least radiation had a significantly lower cancer death rate than the controls (p <0.01), the authors' conclusion is misleading. Contrary to their stated conclusion, their data exhibit hormesis. The threshold was about 6 cSv.
FIGURE 6.
Cumulative cancer death rates in Japanese bomb victims. Numbers on the right list thousands of persons for each dose (Shimizu et al., 1989).
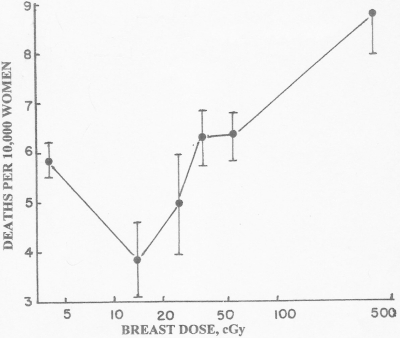
More ugly comes from Canada. Although not mentioned in the abstract, introduction, discussion, or conclusion of their paper, the data of Miller and associates (Fig. 7) showed radiation hormesis in 31,700 women examined for tuberculosis with fluoroscopy (Miller et al., 1989). Low dose irradiation significantly decreased (p <0.01) the breast cancer death rate. The authors did not present a dose-response curve, and reported a conclusion that was inconsistent with their data: “The data were most consistent with a linear dose-response relation.” and “The evidence … indicates that the most appropriate form of the dose-response relation is a simple linear one.” Both statements imply that all radiation was harmful; the authors gave no hint that low dose irradiation (<30 cGy) was beneficial.
FIGURE 7.
Breast cancer deaths in women treated for tuberculosis. Breast cancer death rates of Canadian women repeatedly X-rayed by fluoroscopy to monitor lung-collapse therapy for tuberculosis (Miller et al., 1989). One standard deviation is displayed.
DISCUSSION AND CONCLUSIONS
Radiation hormesis is a very general phenomenon; it extends from bacteria, through plants, insects, invertebrates, and mammals (Luckey, 1980). Low doses of physical, chemical, and biological agents evoke hormesis. Essential nutrients, antimetabolites, and other toxicants are hormetic (Luckey, 1959). Most drugs which have been tested in the low dose range exhibit hormesis (Townsend and Luckey, 1960). Hormesis with heavy metals has been documented (Luckey et al., 1975). Biological agents included hormones, wounds, stress, and adaptive cell responses (Luckey, 1999a, 1999b).
As perceived by Hippocrates more than two millennia ago, a given agent is neither toxic nor beneficial. The dose elicits a continuum response from not effective to biopositive, through optimum, and to excess (Luckey, 1977). Acknowledging harm from large doses, the focus here is on the biopositive effects of low dose irradiation. A wealth of data presents irrefutable evidence of the benefits that radiation provides for a great variety of organisms. This includes decreased infections, decreased cancer death rates, and increased fecundity and average lifespan in humans. Many of these benefits are associated with biological stimulation and suppression of genes, enzymes and other proteins that indicate an activated immune system. Some results, especially the debility caused by reducing radiation below average natural background levels in organisms, indicate that ionizing radiation is essential for life. That is good.
The French stand alone in Europe by accepting the benefits of low dose irradiation. France sells electricity from nuclear power plants to other European countries. The French Academy of Sciences and the French National Academy of Medicine unanimously approved a definitive statement which accepts radiation hormesis (Aurengo et al., 2005).
Some Japanese radiobiologists realize that the abundant data on radiation hormesis support the use of low dose irradiation as an agent for health. Many of the Japanese medical profession rejects “all radiation is harmful” and accept low dose irradiation to “live in harmony with nature”.
In China, an extensive epidemiologic study of two groups of peasants who received different amounts of ambient radiation indicated that low levels of irradiation may be beneficial (Wei, 1997). Intensive studies of the intricacies of a radiation activated immune system provide support for that conclusion (Liu, 1998). China plans to build many new nuclear power plants in the near future (Muckerheide, 2005).
The bad is epitomized by the U. S. government with lost opportunities for industrial development due to unreasonable regulations which restrict radiation exposures for industrial workers to near ambient levels. The lack of popular acceptance of nuclear power is attributable to misleading information provided by the BEIR, NCRP, and ICRP committees which consistently and deliberately fail to consider confirmed scientific reports showing the biopositive effects of low dose irradiation. These restrictions contribute to potential devastation due to insufficient energy in the United States and the world.
The bad is misspent hundreds of billions of taxpayer and consumer dollars in industry and government, millions of needless cancer deaths, and a damaged nation (Muckerheide and Rockwell, 1997). This “science cancer” extends to the National Research Council which consistently appoints biased people to the BEIR committee. The bad includes those foreign governments which capitulated to the deceptions promulgated by the BEIR, NCRP committees, and the United States government agencies (such as the EPA). Misinformation is the hallmark of radiation hormesis.
The health of many people is jeopardized by the refusal of our medical profession to recognize the benefits of low dose irradiation. For example, antibiotic resistance has turned previously accepted medical practice for diabetic patients, effective and painless treatment with low dose irradiation, into 19th century surgery to amputate limbs with the accompanying pain and poor survival rates (Luckey, 2005a).
Studies of cancer death rates in nuclear industries involved over 7 million person-years (Luckey, 1997a, 1999a). The results showed that low dose irradiation decreased cancer death rates by almost 50%. If families of cancer patients realized this, they would insist upon access to low dose irradiation. And they would want to prosecute BEIR and NCRP committee members for their decades of erroneous information causing needless suffering and deaths.
There is a hidden bad. Considerable information indicates that we live in a partial deficiency of ionizing radiation. Nuclear wastes could provide safe radiation spas throughout the world (Luckey, 1995a, 1995b, 2004). Low dose irradiation could be provided in hospitals as a public health measure. If we had 50 times more radiation than we now receive, we would reach a new plateau of health (Luckey, 1999a, 1999b).
The most ugly is the consistent failure by our BEIR and NCRP committees, and comparable international committees, to report on the voluminous data showing beneficial effects of low dose irradiation. Each year, millions of taxpayer dollars go to these committees, researchers, and consultants who use “meta analysis” and contrived units of measurement (working level months) to confuse the issues. The few examples provided above indicate how these committees, and many radiobiologists, mislead our government and the public. Some of their statements appear to be scientific falsifications and fabrications. Thousands of creditable results have been reviewed (Luckey, 1980, 1991, Muckerheide, 2001). Documentation of the ugly is good; it illustrates one half-century of producing incomplete and misleading statements by some radiobiologists and the lack of credibility of the BEIR and NCRP committees. Calabrese reviewed the history of the rejection of the concept of hormesis: “The effects of this century-long conflict have been as destructive as they have been overlooked, …” (Calabrese, 2005).
ACKNOWLEDGMENT
It is a pleasure to acknowledge the help of Donna Luckey and Sara Jane Luckey in the preparation of this manuscript.
REFERENCES
- Aurengo A., Averbeck D., Bonnin A., LeGuen B., Masse R., Monier R., Tubiana M., Valleron A.J., de Vathaire F. Dose-Effect Relationships and Estimation of the Carcinogenic Effects of Low Doses of Ionizing Radiation. Paris: Academies of Sciences and Medicine; 2005. [Google Scholar]
- Becker K. A loss of innocence? Rad Protect Dosimetry. 1995;59:234–235. [Google Scholar]
- Becker K. Health effects of high radon environments in central Europe: another test for the LNT hypothesis. Nonlinear Biol. Toxicol Med. 2005;1:3–35. doi: 10.1080/15401420390844447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIR IV Committee . The Health Effect of Radon and Other Internally Deposited Alpha-Emitters. Washington, DC: The National Academy Press; 1988. [PubMed] [Google Scholar]
- BEIR VI Committee . Health Effects of Exposure to radon. Washington, DC: The National Academy Press; 1999. [PubMed] [Google Scholar]
- BEIR VII - Phase 2 Committee . Health Risks from Exposure to Low Levels of Ionizing Radiation. Washington, DC: The National Academy Press; 2005. [PubMed] [Google Scholar]
- Berk L.D., Hodes P.J. Roentgen therapy for infections: an historical review. Yale J Biol Med. 1991;64:155–165. [PMC free article] [PubMed] [Google Scholar]
- Bogoljubov W.M. Clinical aspects of radon therapy in the U.S.S.R. J Phys Med Balneol Med Klimatol. 1988;17:59–70. [Google Scholar]
- Borak J. Theories on the effectiveness of roentgen therapy in inflammatory conditions. Radiology. 1944;42:2249–254. [Google Scholar]
- Brucer M. A Chronology of Nuclear Medicine. St. Louis: Heritage Foundation; 1990. [Google Scholar]
- Calabrese E. Historical blunders: how toxicology got the dose-response relationship half right. Cell Molec Biol. 2005;51:643–654. [PubMed] [Google Scholar]
- Calabrese E., Baldwin L. Radiation hormesis: its historical foundations as a biological hypothesis. Belle Newsletter. 1999;8:2–37. doi: 10.1177/019262339902700207. [DOI] [PubMed] [Google Scholar]
- Chen W.L., Luan Y.C., Shici M.C., Chen S.T., Kung H.T., Soong K.L., Yeh Y.C., Chou S.H., Mong S.H., Wu J.T., Sun C.P., Deng W.P., Wu M.F., Shen M.L. Is chronic radiation an effective prophylaxis against cancer? J Am Phys Surg. 2004;9:6–10. [Google Scholar]
- Cohen B.L. Test of the linear no-threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen B.L. Radon in air. In: Lehr J.H., Lehr J.K., editors. Standard Handbook of Environmental Science, Health and Technology. New York: McGraw-Hill; 2000. pp. 15.7–15.19. [Google Scholar]
- Conter A., Dupouy D., Planel M. Demonstration of a biological effect of natural ionizing radiation. Intern J Rad Biol. 1982;43:421–432. doi: 10.1080/09553008314550481. [DOI] [PubMed] [Google Scholar]
- Cullen T.L., Franca E.P., editors. International Symposium on Areas of High Natural Radioactivity. Rio de Janeiro: Acad. Brasileira de Ciencias; 1977. [Google Scholar]
- Cuttler J.M. Disinfecting wounds with radiation. Ann. Conf. Can. Nucl. Soc. 2002:1–10. [Google Scholar]
- Desjardins A.U. Radiotherapy for inflammatory conditions. J Am Med Assoc. 1931;98:401–408. [Google Scholar]
- Desjardins A.U. The action of Roentgen rays on inflammatory conditions. Radiol. 1942;38:274–280. [Google Scholar]
- Deetjen P. Biological and therapeutical properties of radon. In: Katase A., Shimo M., editors. Radon and Thoron in the Human Environment. Singapore: WorldScientific; 1998. pp. 515–522. [Google Scholar]
- Gold T. The Deep Hot Biosphere. New York: Springer Verlag; 1998. [Google Scholar]
- Gould R.F. Radiation Chemistry. volume 1. Washington: American Chemical Assoc; 1968. [Google Scholar]
- Gustavsson A., Osterman B., Cavallin-Stahl E. Systematic overview of radiation therapy effects in non-Hodgkin's lymphoma. Acta Oncol. 2003;42:605–619. doi: 10.1080/02841860310014435. [DOI] [PubMed] [Google Scholar]
- Hattori S. State of research and perspective on radiation hormesis in Japan. Intern J Occup Med Toxicol. 1994;3:203–217. Also BELLE Newsletter 3:1–4. [Google Scholar]
- Heidenhain, L. (1026). Roentgenbestrahlung und Entzundung. Strahlenther 24:337–51.
- Hellstrom I., Hellstrom K.E. Antitumor effect of whole body X-irradiation: possible role of X-ray sensitive T suppressor cell population. Transplant Proc. 1979;11:1073–1079. [PubMed] [Google Scholar]
- Ina Y., Sakai K. Prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice. Rad Res. 2004;161:168–173. doi: 10.1667/rr3120. [DOI] [PubMed] [Google Scholar]
- Ina Y., Sakai K. Suppression of thymic lymphoma induction by life-long low dose rate irradiation accompanied by immune activation in C57BL/6 mice. Rad Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Jaworowski Z. Hormesis: the beneficial effects of radiation. 21st Century. 1994;7:22–26. [Google Scholar]
- Jovanovic S., (2005). Univ. Nis, Serbia, personal communication.
- Kaplan I.I. Clinical Radiation Therapy. New York: P. B. Hoeber; 1949. [Google Scholar]
- Kelly J.F., Dowell D.A. Roentgen Treatment of Infections. Chicago: The Yearbook Publishers, Inc.; 1942. [Google Scholar]
- Lewis P., (2005). Personal communication, email: lewis@radonmine.com.
- Lortet L., (1997). p. 680, The Lancet, March 6.
- Liu S.Z. Biological defense and adaptation induced by low dose radiation. Human & Ecol Risk Assess J. 1998;4:1217–1254. [Google Scholar]
- Luckey T.D. Antibiotics in nutrition. In: Goldberg H.S., editor. Antibiotics, their Chemistry and Non-Medical Uses. New York: D. Van Nostrand Co., Inc.; 1959. pp. 174–321. [Google Scholar]
- Luckey T.D. Activity spectrum of ingested toxicants. In: Rechcigl M., editor. Comparative Animal Nutrition. vol. 2. Basel: 1977. pp. 144–178. [Google Scholar]
- Luckey T.D. Hormesis with Ionizing Radiation. Boca Raton: CRC Press, Inc.; 1980. [Google Scholar]
- Luckey T.D. Radiogenic metabolism. Am J Clin Med. 1980;33:2544. [Google Scholar]
- Luckey T.D. Radiation Hormesis. Boca Raton: CRC Press, Inc.; 1991. [Google Scholar]
- Luckey, T. D. (1993). Is EPA carcinogenic? Health Phys. Soc. Newsl. Nov. 1993.
- Luckey T.D. Live in harmony with ionizing radiation. In: Zu X., Liu S-Z., editors. International Symposium on Biological Effects of Low Level Exposures to Radiation and Related Agents. Changchung, China: Norman Bethume Univ; 1995. pp. 40–71. [Google Scholar]
- Luckey T.D. Radiation hormesis: radioactive waste for health. Am Nucl Soc Trans. 1995;73:39–40. [Google Scholar]
- Luckey T.D. Low dose irradiation reduces cancer death rates. Rad Protect Manag. 1997;14:58–64. [Google Scholar]
- Luckey T.D. Estimation of a minimum yearly allowance (MYRA) J. Clean Technol. Environ. Toxicol. 1997;6:239–252. [Google Scholar]
- Luckey T.D. Nurture with ionizing radiation; a provocative hypothesis. Nutr and Cancer. 1999;34:1–11. doi: 10.1207/S15327914NC340101. [DOI] [PubMed] [Google Scholar]
- Luckey T.D. Radiation hormesis overview. Rad Protect Manag. 1999;16:22–34. [Google Scholar]
- Luckey T.D. Radiobiology deceptions reject health. Proc. ICONE. 2000;8:11–49. Baltimore. [Google Scholar]
- Luckey T.D. Nuclear triage and the dirty bomb. Rad. Protect. Manag. 2004;20:11–18. [Google Scholar]
- Luckey T.D. Low dose irradiation therapy. RSO. 2005;9:14–19. [Google Scholar]
- Luckey T.D. Low dose irradiation for gingivitis. Med Hypotheses. 2005 doi: 10.1016/j.mehy.2005.10.020. web. [DOI] [PubMed] [Google Scholar]
- Luckey T.D., Venugopal P., Hutcheson D. Heavy Metal Toxicity, Safety and Hormology. Stuttgart: George Thieme, Publ.; 1975. [Google Scholar]
- Miller A.B., Howe G.R., Sherman G.J., Lindsay J.P., Yaffe M.J., Dinner P.J., Risch H.A., Preston D.L. Mortality from breast cancer after irradiation during fluoroscopic examination in patients being treated for tuberculosis. New England J Med. 1989;321:11285–11288. doi: 10.1056/NEJM198911093211902. [DOI] [PubMed] [Google Scholar]
- Mine M. Apparently beneficial effect of low to intermediate doses of A-bomb radiation on human lifespan. Yokohama Med Bull. 1991;422:2–3. doi: 10.1080/09553009014552341. [DOI] [PubMed] [Google Scholar]
- Macklin R. The great radium scandal. Sci Am. 1993;269:94–99. doi: 10.1038/scientificamerican0893-94. [DOI] [PubMed] [Google Scholar]
- Muckerheide J. Low-Level Radiation Health Effects: a Compilation of Data and Programs. Needham, MA: RSH, Inc.; 2001. [Google Scholar]
- Muckerheide J. How to build 6, 000 nuclear plants by 2050. 21st Century Sci Technol. 2005;18:36–51. [Google Scholar]
- Muckerheide J., Rockwell T. The hazards of U. S. Policy on low-level radiation. 21st Century Sci Technol. 1997;10:126–132. [Google Scholar]
- NEA Committee . Chernobyl: Ten Years on Radiological and Health Impact. Paris: Nuclear Energy Agency, OCED (Organization for Economic Co-Operation and Development); 1995. [Google Scholar]
- Pierce D.A., Preston D.L. Cancer risks at low doses among A-bomb survivors. RERF Update. 2001;12:15–17. [Google Scholar]
- Polycove M., Feinendegen L. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Human and Exptl Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Rowland R.E., Stehney A.F., Lucas H.F. Dose-response relationships for female radium dial workers. Radiat Res. 1978;76:368–383. [PubMed] [Google Scholar]
- Rowland R.E. Bone sarcoma in humans induced by radium: a threshold response? Proc European Soc Radiat Biol. 1997;32:331–338. [Google Scholar]
- Ruda V.P., Kuzin A.M. The occurrence of hormesis during gamma radiation of developing rat pups. Radiobiologica. 1991;33:345–347. [PubMed] [Google Scholar]
- Sakamoto K., Myonin M., Hosor Y., Ogawa Y., Nemoto K., Takai Y., Kakuto Y., Yamada S., Watabe N. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9:161–175. [Google Scholar]
- Sakamoto K., Myojin J. Fundamental and clinical studies on tumor control by total body irradiation. Am Nucl Soc Trans. 1996;75:404–5. [Google Scholar]
- Salak K. Mining for miracles. Nat Geo. 2003;205:118–122. [Google Scholar]
- Shrader W. Experiments with X rays upon germs. Elect Engineer. 1896;22:176–7. [Google Scholar]
- Shrader W. Biological effects of X rays. Elect Engineer. 1896;22:183. [Google Scholar]
- Shimizu Y., Kato H., Schull, Mabuchi K. Dose-response analysis among atomic-bomb survivors to low-level radiation. In: Sugaahara T., Sagan L., Aoyama T., editors. Low Dose Irradiation and Biological Defense Mechanisms. Amsterdam: Excerpta Medica; 1992. pp. 71–74. [Google Scholar]
- Townsend J.F., Luckey T.D. Hormologosis in pharmacology. J. Am. Med. Assoc. 1960;173:44–48. doi: 10.1001/jama.1960.73020190007010. [DOI] [PubMed] [Google Scholar]
- UNSCEAR . Sources and effects of ionizing radiation, Annex B. Adaptive responses to radiation in cells and organisms. New York: United Nations; 1995. [Google Scholar]
- Wei L. High background radiation area. In: Wei L, Sugahara T, Tao Z, editors. High Levels of Natural Radiation. Amsterdam: Elsevier; 1997. pp. 1–14. 58–59, and 63–66. [Google Scholar]