Abstract
Dose-response curves for various low-LET radiation sources have consistently been demonstrated to be J-shaped for the cancer-relevant endpoint of neoplastic transformation in vitro. Most of these studies have been performed where the radiation has been delivered at intermediate to high dose-rates (30–3000 mGy/min), where the threshold dose for induction of neoplastic transformation is around 100–200 mGy. Below these doses, the transformation frequency is less than that seen spontaneously, indicative of a hormetic effect. More recently, data have been obtained for low dose rates (<0.5 mGy/min) of low-LET radiation, and again hormetic effects are apparent but with threshold doses now being >1000 mGy. Similar trends have been reported in animal experiments as well as in human epidemiologic studies. Indeed, the relative risks for induction of neoplastic transformation in vitro in the dose range 1 to 1000 mGy agree well with those for incidence of radiation-induced breast cancer and leukemia in humans. These findings support the notion that the endpoint of neoplastic transformation in vitro is a plausible endpoint to not only study mechanisms involved in response to low doses of radiation, but also to provide information of potential importance to risk assessment.
Keywords: low dose, radiation, neoplastic transformation, hormesis, risk assessment
RELEVANCE OF IN VITRO ASSAYS
The in vitro assay of neoplastic transformation has a long history of providing quantitative and mechanistic data that parallels that found in vivo (Little 1989). Such findings relate to the effects of dose, dose-rate, radiation quality, and effects of chemical modifiers and as such are regarded as being of value to the study of radiation carcinogenesis. This is despite the obvious microenvironmental limitations and lack of an immune response compared to the in vivo situation. Another perceived limitation is the refractory nature of primary human cell cultures to radiation-induced transformation to the neoplastic phenotype. Even following immortalization, such cells are difficult to neoplastically transform with radiation and usually require repeated high radiation doses over a period of time. Such systems are of value in assessing molecular and cellular characteristics of the radiation-induced tumorigenic cells but are not suitable for quantitative studies (e.g. Hei et al. 2001). The major workhorse assay in the field of quantitation of radiation-induced neoplastic transformation in vitro has been the C3H 10T1/2 mouse embryo fibroblast system. Another assay that has recently been gaining wider acceptance is the HeLa × skin fibroblast human hybrid cell assay (Redpath et al. 1987, Mendonca et al. 1992). This assay has some advantages, both practical and mechanistically, over the C3H 10T1/2 system. Firstly, the assay takes 21–25 days to go to completion compared to up to 60 days for the C3H 10T1/2 assay. Secondly, the neoplastic phenotype has a cell surface molecular marker that can be used to identify foci of tumorigenic cells, as compared to the morphologic changes used with C3H 10T1/2 cells. Thirdly, the mechanism involved in the neoplastic transformation is the loss of putative tumor suppressor loci located on chromosomes 11 and 14 (Mendonca et al. 1998). Both the C3H 10T1/2 and the human hybrid cells should be considered as partway down the pathway from normal to tumorigenic, i.e. they are preneoplastic. The relevance of use of such cells could also be questioned. However, since most humans have burdens of preneoplastic cells in their body, they could be considered to be a particularly relevant target for carcinogens, including radiation.
ADAPTIVE RESPONSE
The ability of a low dose of radiation, say 1 to 100 mGy, to ameliorate the effect of a subsequent high radiation dose, say several Gy, is well described for a variety of in vitro and in vivo endpoints and has historically been described as an adaptive response. However, an adaptive response can also be observed following a low dose exposure alone, without using a high dose challenge to allow its detection. For example, it is conceivable that such low-dose responses could impact the shape of dose response curves at low doses, including for the endpoint of neoplastic transformation in vitro, where, like for human cancer, there is a certain background incidence. In addition, such low adapting doses can alter the the latency period for the appearance of spontaneous tumors in vivo (Mitchel et al. 2003). Mechanistically, adaptive responses have been linked to radiation-induced protein synthesis, including the upregulation of DNA repair and cellular antioxidants, (Wolff 1998 and references therein), the promotion of apoptosis (Cregan et al. 1999), and the activation of immunological networks (Ina and Sakai 2005a).
SUPPRESSIVE EFFECTS OF LOW DOSES OF LOW-LET RADIATION FOR NEOPLASTIC TRANSFORMATION IN VITRO
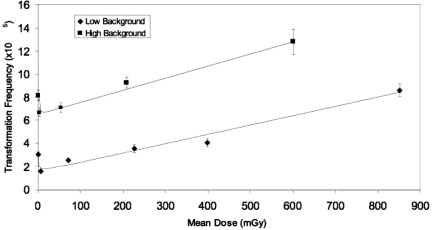
Azzam et al. (1996) demonstrated that low doses of 1, 10, and 100 mGy of Co-60 gamma irradiation suppressed the transformation frequency of C3H 10T1/2 cells to levels less than that seen spontaneously. This was subsequently verified for a dose of 10 mGy of Cs-137 gamma radiation in the human hybrid cell assay (Redpath and Antoniono 1998). Full dose-response curves were then developed for a series of low-LET sources including Cs-137 gamma rays, 60 kVp x-rays, 28 kVp x-rays and 232 MeV protons (Redpath et al. 2001, Redpath et al. 2003b, Ko et al. 2004, Elmore et al. 2005, Ko et al. 2006). These dose-response curves were J-shaped, and consistently demonstrated suppression at low doses with thresholds between 100 and 200 mGy. These data have now been combined for analysis. It is realized that while all the data are for low-LET radiation sources, they are for sources of different energy that potentially have different biological effectiveness. However, even given this limitation, it was felt worthwhile to do a combined analysis to see if the low dose-suppression seen in individual studies still holds when that data are combined. Since these experiments were performed over a period of years using different batches of serum, and since serum batch is well known to influence background frequency, this combination had to be done for two groups separated by level (‘low’ and ‘high’) of spontaneous background frequencies. Details of these analyses are shown in Tables 1 and 2 and the corresponding dose-response curves are shown in Figure 1. As is shown, the combined data clearly show a hormetic effect with J-shaped curves for both ‘low’ and ‘high’ background frequencies. The most significant suppression was apparent for the lowest dose cohort (<10 mGy) for both groups and the threshold was between 100 and 200 mGy for both groups. It is of importance to note that the suppressive effect of low doses of low-LET radiation on neoplastic transformation in vitro has been demonstrated over a wide range of background frequencies from 1.8 × 10−3 (Azzam et al. 1996) to 2.2 × 10−5 (Redpath et al. 2001) and indicates that the hormetic effect is independent of genetic stability of the target cell population.
TABLE 1.
Summary of low background experiments to date on the effect of low doses of low-LET radiation on the neoplastic transformation of HeLa × skin fibroblast hybrid cells.
| Dose Cohort, mGy | Paper No.∗ | Mean Dose (mGy) | No. of Surviving cells S(×105) | No. of Foci | No. of Flasks, N | No. of Flasks without Foci, n | Transformation Frequency (×10−5) | ± SE (×10−5) | 95% Confidence Intervals (×10−5) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 | 0 | 15.66 | 73 | 376 | 315 | 4.25 | 0.54 | |
| 2 | 29.32 | 65 | 762 | 699 | 2.24 | 0.28 | |||
| 3 | 35.07 | 106 | 1297 | 1198 | 2.94 | 0.30 | |||
| 4 | 25.44 | 92 | 669 | 582 | 3.66 | 0.39 | |||
| sum | 105.49 | 336.00 | 3104.00 | 2794.00 | 3.10 | 0.18 | 2.74, 3.45 | ||
| 1–10 | 1 | 5.1 | 15.41 | 36 | 377 | 346 | 2.10 | 0.38 | |
| 2 | 25.28 | 37 | 708 | 672 | 1.46 | 0.24 | |||
| 3 | 31.23 | 28 | 1173 | 1148 | 0.81 | 0.16 | |||
| 4 | 11.5 | 39 | 287 | 249 | 3.54 | 0.58 | |||
| sum | 83.42 | 140 | 2545 | 2415 | 1.60 | 0.14 | 1.32, 1.88 | ||
| 11–100 | 2 | 69.2 | 16.08 | 25 | 426 | 401 | 1.60 | 0.32 | |
| 3 | 28.67 | 63 | 960 | 900 | 2.16 | 0.28 | |||
| 4 | 29.64 | 99 | 879 | 781 | 3.51 | 0.35 | |||
| sum | 74.39 | 187 | 2265 | 2082 | 2.57 | 0.19 | 2.19, 2.94 | ||
| 101–300 | 2 | 226.7 | 8.62 | 33 | 216 | 188 | 3.48 | 0.66 | |
| 3 | 8.38 | 27 | 288 | 262 | 3.25 | 0.64 | |||
| 4 | 13.16 | 54 | 347 | 300 | 3.84 | 0.56 | |||
| sum | 30.16 | 114 | 851 | 750 | 3.56 | 0.35 | 2.85, 4.27 | ||
| 301–500 | 2 | 397.7 | 11.76 | 45 | 288 | 248 | 3.66 | 0.58 | |
| 3 | 8.81 | 44 | 288 | 248 | 4.89 | 0.77 | |||
| 4 | 15.71 | 67 | 479 | 421 | 3.94 | 0.52 | |||
| sum | 36.28 | 156 | 1055 | 917 | 4.08 | 0.35 | 3.38, 4.77 | ||
| 501–1000 | 2 | 850 | 7.34 | 47 | 192 | 151 | 6.28 | 0.98 | |
| 4 | 11.8 | 77 | 320 | 260 | 5.63 | 0.73 | |||
| sum | 32.48 | 357 | 956 | 713 | 8.63 | 0.56 | 7.52, 9.74 |
Paper 1 = Redpath and Antoniono, 1998; Paper 2 = Redpath et al., 2001; Paper 3 = Redpath et al., 2003; Paper 4 = Ko et al., 2006.
TABLE 2.
Summary of high backround experiments to date on the effect of low doses of low-LET radiation on the neoplastic transformation of HeLa × skin fibroblast hybrid cells.
| Dose Cohort | Paper No.∗ | Mean Dose (mGy) | No. of Surviving Cells S(×105) | No. of Foci | No. of Flasks, N | No. of Flasks without Foci, n | Transformation Frequency (×10−5) | ± SE (×10−5) | 95% Confidence Intervals (×10−5) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 5 | 0 | 25.13 | 229 | 672 | 497 | 8.07 | 0.61 | |
| 6 | 14.99 | 152 | 442 | 333 | 8.35 | 0.80 | |||
| sum | 40.12 | 381.00 | 1114.00 | 830.00 | 8.17 | 0.49 | 7.20, 9.14 | ||
| 5 | 2.7 | 28.88 | 223 | 900 | 734 | 6.35 | 0.49 | ||
| 6 | 28.73 | 253 | 882 | 702 | 7.01 | 0.52 | |||
| sum | 57.61 | 476 | 1782 | 1436 | 6.68 | 0.36 | 5.96, 7.40 | ||
| 11–100 mGy | 5 | 53.7 | 33.48 | 243 | 912 | 728 | 6.14 | 0.45 | |
| 6 | 14.7 | 164 | 444 | 325 | 9.42 | 0.87 | |||
| sum | 48.180 | 407 | 1356 | 1053 | 7.12 | 0.41 | 6.30, 7.94 | ||
| 101–300 mGy | 5 | 208 | 37.79 | 387 | 978 | 686 | 9.18 | 0.54 | |
| 6 | 13.83 | 160 | 444 | 330 | 9.53 | 0.90 | |||
| sum | 51.620 | 547 | 1422 | 1016 | 9.26 | 0.46 | 8.34, 10.18 | ||
| 501–1000 cGy | 6 | 600 | 13.34 | 233 | 444 | 302 | 12.83 | 1.08 | |
| sum | 13.340 | 233 | 444 | 302 | 12.83 | 1.08 | 10.66, 14.99 |
Paper 5 = Ko et al., 2004; Paper 6 = Elmore et al., 2005.
FIGURE 1.
Neoplastic transformation frequency as a function of low-LET radiation dose: An analysis of combined data from several independent studies using the human hybrid cell assay. The analyses are stratified by level of spontaneous background into two groups (“low” and high”). For further detail see Tables 1 and 2.
Mechanistic studies indicate that multiple mechanisms are likely involved in a dose-dependent fashion. These include the upregulation of DNA repair, and the hyper-radiosensitivity to radiation-induced cell death of a transformation prone subpopulation (Pant et al. 2003; Redpath, Short et al. 2003a).
Mathematical modeling of our neoplastic transformation data has invoked the concept of protective apoptosis-mediated (PAM) death of cells destined to become neoplastically transformed to account for the protective effect against neoplastic transformation seen at low doses (Schoellenberger et al. 2002, Scott et al. 2003, Scott 2005)
Bystander effects due to factors excreted into the extracellular medium do not appear to play a role in this assay although bystander effects as a consequence of gap junction intracellular communication may do so in a way which partially offsets effects due to an adaptive response (Ko et al. 2006). Recent low dose-rate studies (Elmore et al 2006) have shown that the suppressive effects still exist at low doses and the threshold dose is increased as the dose-rate is decreased.
IN VIVO RESPONSES AT LOW DOSES
Dose-response curves consistent with a threshold effect have also been found in animal studies (Ullrich and Storer 1979) and low doses have been found to increase the latency period for tumor formation in both normal and cancer prone mice (Mitchel et al. 1999; Mitchel et al. 2003). Low dose-rate studies in animals also strongly support the possibility of dose thresholds (Ullrich and Storer 1979). Recent studies have implicated immune activation in the suppression of cancer induction in mice by chronic low dose-rate irradiation (Ina and Sakai 2005a, Ina et al. 2005). In addition, chronic low dose-rate irradiation has been shown to prolong the lifespan of mice genetically engineered to be susceptible to multiple severe diseases, again through immunological activation (Ina and Sakai 2005b).
COMPARISON WITH EPIDEMIOLOGIC FINDINGS
Dose-response curves for radiation induced cancer in adult humans can often be equally well fitted with a threshold, a linear-quadratic or a linear model emphasizing the difficulty in assessing what is really happening at low doses (≤ 100 mSv) from epidemiologic data. Furthermore, when analyses are performed on incidence of (or mortality from) all solid cancers, as is often the case for A-bomb survivors, the variability of response in terms of individual tumor types is obscured. For example, Preston et al. (2003) have shown differences in mortality risk estimates for a variety of radiation-induced cancers. While the data for radiation-induced leukemia in the A-bomb survivors can be fit with a no-threshold model, it is also not possible to rule out the existence of a threshold in the range of 100 mSv (Little and Muirhead 1998). Indeed, the low-dose data from this study are very suggestive of a J-shaped, hormetic-type, dose-response (see UNSCEAR 2000). The same is true for radiation-induced breast cancer in humans. The paper on the pooled analysis of eight cohorts for radiation effects on breast cancer risks represents a valuable compilation of data for high and low dose-rate exposure (Preston et al. 2002). For doses <100 mGy delivered at high dose-rates, the relative risks trend, although not significantly so, to values <1. This is even more apparent for low dose-rate exposures where the relative risks are <1 even for exposures in dose cohorts up to 1000 mGy. Indeed, in one of the original papers on the low-dose cohorts the authors state that “it was the contribution of subjects with breast doses >1 Gy that produced a positive association between dose and subsequent breast cancer risk” (Lundell et al. 1996). This is but one illustration of a general concern that while epiemiologic data can often be fit to a linear no-threshold (LNT) model, such fitting is often heavily driven by the high dose data points and this can tend to obscure what is happening at low doses.
We have previously observed that relative risks for leukemia and breast cancer incidence and mortality agree well with those determined by our in vitro transformation assay for intermediate to high dose-rate low-LET radiation (Redpath et al. 2001). We now have data showing a similar excellent agreement for low dose-rate low-LET radiation when comparing relative risk for breast cancer incidence with that for neoplastic transformation in vitro (Elmore et al. 2006). The data indicate that for low to high dose-rates (1 to 2000 mGy/min) a threshold dose of 100 mGy cannot be ruled out and that for very low dose-rates (< 0.5 mGy/min) this threshold could well exceed 500 to 1000 mGy Furthermore, for both endpoints the existence of a hormetic effect cannot be statistically ruled out. A recent joint report of the French Academie des Sciences and Academie Nationale de Medecine also concluded that the LNT model could greatly overestimate the carcinogenic risks associated with low doses (< 100 mGy) of low LET radiation (Tubiana and Aurengo 2006). On the other hand the recent report from the U.S. National Academy of Sciences (BEIR VII PHASE II 2006) concluded that there is no compelling evidence to indicate a dose threshold below which the risk of tumor induction is zero. The fact that two distinguished committees came to opposite conclusions is largely a reflection of their differing interpretations of recent biological data, including some of that referred to in this paper. Hopefully, further research into low-dose effects will eventually resolve this difference.
ACKNOWLEDGMENTS
The support of the Office Science (BER), U.S. Department of Energy Low Dose Radiation Program Grant Numbers DE-FG07-99ER62876, DE-FG03-02ER63309 and DE-FG02-03ER63648, the Phi Beta Psi Sorority, research laboratory members and collaborators are gratefully acknowledged.
REFERENCES
- Azzam EI, De Toledo SM, Raaphorst GP, Mitchel R. Low dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- BEIR VII PHASE II, 2006. Health risks from exposure to low levels of ionizing radiation The National Academies Press, Washington D.C. [PubMed]
- Cregan SP, Brown DL, Mitchel RE. Apoptosis and adaptive response in human lyphocytes. Int J Radiat Biol. 1999;75:1087–1094. doi: 10.1080/095530099139548. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao X-Y, Ko M, Rightnar S, Nelson G, Redpath JL. Neoplastic transformation in vitro induced by low doses of 232 MeV protons. Int J Radiat Biol. 2005;81:291–297. doi: 10.1080/09553000500140324. [DOI] [PubMed] [Google Scholar]
- Elmore E, Lao X-Y, Kapadia R, Redpath JL. The effect of dose-rate on radiation-induced neoplastic transformation in vitro by low doses of low-LET radiation. Radiat. Res. 2006;166:832–838. doi: 10.1667/RR0682.1. [DOI] [PubMed] [Google Scholar]
- Hei TK, Zhao YL, Roy D, Piao CQ, Calaf G, Hall EJ. Molecular alterations in tumorigenic human bronchial and breast epithelial cells induced by high LET radiation. Adv Space Res. 2001;27:411–419. doi: 10.1016/s0273-1177(01)00009-6. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Activation of immunological net work by chronic low-dose-rate irradiation of wild type mouse strains: analysis of immune cell populations and surface molecules. Radiat Res. 2005;81:721–729. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- Ina Y, Sakai K. Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole life irradiation. Radiat Res. 2005;163:418–423. doi: 10.1667/rr3316. [DOI] [PubMed] [Google Scholar]
- Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accomjpanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Lao X-Y, Molloi S, Elmore E, Redpath JL. Neoplastic transformation in vitro following exposure to low doses of mammographic energy x-rays: Quantitative and mechanistic aspects. Radiat Res. 2004;162:646–654. doi: 10.1667/rr3277. [DOI] [PubMed] [Google Scholar]
- Ko M, Lao X-Y, Kapadia R, Elmore E, Redpath JL. Neoplastic transformation in vitro: role of adaptive response and bystander effects. Mut Res. 2006;597:11–17. doi: 10.1016/j.mrfmmm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Little JB. The relevance of cell transformation to carcinogenesis in vivo. In: Baverstock KF, Strather JW, editors. Low Dose Radiation: Biological Bases of Risk Assessment. London: Taylor and Francis; 1989. pp. 396–413. [Google Scholar]
- Little MP, Muirhead CR. Curvature in the cancer mortality dose –responses in Japanese atomic bomb survivors: absence of evidence of threshold. Int J Radiat Biol. 1998;74:471–480. doi: 10.1080/095530098141348. [DOI] [PubMed] [Google Scholar]
- Lundell M, Mattsson A, Hakulinen T, Holm L-E. Breast cancer after radiotherapy in infancy. Radiat. Res. 1996;145:225–230. [PubMed] [Google Scholar]
- Mendonca MS, Antoniono RJ, Sun C, Redpath JL. A simplified and rapid staining method for the HeLa × skin fibroblast human hybrid cell neoplastic transformation assay. Radiat Res. 1992;131:345–350. [PubMed] [Google Scholar]
- Mendonca MS, Howard K, Fasching CL, Farrington DL, Desmond LA, Stanbridge EJ, Redpath JL. Loss of suppressor loci on chromosomes 11 and 14 may be required for radiation-induced neoplastic transformation of HeLa × skin fibroblast human cell hybrids. Radiat. Res. 1998;149:246–255. [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, McCann RA, Boreham DR. The adaptive response modifies latency for radiation-induced myeloid leukemia in CBA/H mice. Radiat Res. 1999;152:273–279. [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pant MC, Liao X-Y, Lu Q, Molloi S, Elmore E, Redpath JL. Mechanisms of suppression of neoplastic transformation in vitro by low doses of low LET radiation. Carcinogenesis. 2003;24:1961–1965. doi: 10.1093/carcin/bgg172. [DOI] [PubMed] [Google Scholar]
- Preston D, Mattsson A, Holmberg E, Shore R, Hildreth N, Boice J. Radiation effects on breast cancer risk: A pooled analysis of eight cohorts. Radiat Res. 2002;158:220–235. doi: 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Preston D, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Antoniono RJ. Induction of an adaptive response against low dose gamma radiation. Radiat Res. 1998;149:517–520. [PubMed] [Google Scholar]
- Redpath JL, Sun C, Colman M, Stanbridge EJ. Neoplastic transformation of human hybrid cells by gamma radiation: A quantitative assay. Radiat Res. 1987;110:468–472. [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, Christie C, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: Evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Short SC, Woodcock M, Johnston PJ. Low dose reduction in transformation frequency compared to unirradiated controls: the role of hyper-radiosensitivity to cell kill death. Radiat Res. 2003;159:433–436. doi: 10.1667/0033-7587(2003)159[0433:ldritf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Liao X-Y, Molloi S, Elmore E. Low doses of diagnostic energy x-rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003;79:235–240. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Scott BR, Walker DM, Tesfaigi Y, Schollnberger H, Walker V. Mechanistic basis for non-linear dose-response relationships for low-dose radiation-induced stochastic effects. Nonlinearity in Biol Toxicol and Med. 2003;1:93–122. doi: 10.1080/15401420390844492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Stochastic thresholds: A novel explanation of non-linear dose-response relationships for stochastic radiobiological effects. Dose-Response. 2005;3:547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the Joint Report of the Academies des Sciences (Paris) and of the Nationale Academie de Medecine. Int J Low Radiation. 2006;2:1–19. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Ullrich RL, Storer JB. Influence of _ irradiation on the development of neoplastic disease in mice. III. Dose-rate effects. Radiat Res. 1979;80:325–342. [PubMed] [Google Scholar]
- UNSCEAR, 2000. Sources and effects of ionizing radiation Vol. II: Effects. United Nations Scientific Committee on the Effects of Atomic Radiations, Report to the General Assembly, United Nations, New York.
- Wolff S. The adaptive response in radiobiology: evolving insights and implications. Env Health Perspect. 1998;106(Suppl. 1):277–283. doi: 10.1289/ehp.98106s1277. [DOI] [PMC free article] [PubMed] [Google Scholar]