Abstract
Indirect effects may contribute to the efficacy of radiotherapy by sterilizing malignant cells that are not directly irradiated. However, little is known of the influence of indirect effects in targeted radionuclide treatment. We compared γ-radiation-induced bystander effects with those resulting from exposure to three radiohaloanalogues of meta-iodoben-zylguanidine (MIBG): [131I]MIBG (low linear energy transfer (LET) α-emitter), [123I]MIBG (high LET Auger electron emitter), and meta-[211At]astatobenzylguanidine ([211At]MABG) (high LET α-emitter). Cells exposed to media from γ-irradiated cells exhibited a dose-dependent reduction in survival fraction at low dosage and a plateau in cell kill at > 2 Gy. Cells treated with media from [131I]MIBG demonstrated a dose-response relationship with respect to clonogenic cell death and no annihilation of this effect at high radiopharmaceutical dosage. In contrast, cells receiving media from cultures treated with [211At]MABG or [123I]MIBG exhibited dose-dependent toxicity at low dose but elimination of cytotoxicity with increasing radiation dose (i.e. U-shaped survival curves). Therefore radionuclides emitting high LET radiation may elicit toxic or protective effects on neighboring untargeted cells at low and high dose respectively. We conclude that radiophar-maceutical-induced bystander effects may depend on LET and be distinct from those elicited by conventional radiotherapy.
Keywords: Radiopharmaceutical-induced bystander effect
I. INTRODUCTION
When cells are exposed to ionizing radiation, they release poisons which result in death, mutation, chromosomal aberrations or long term genomic instability in neighboring, unirradiated cells (Mothersill and Seymour 2001; Mothersill and Seymour 2004; Lyng et al 2002; Lorimore and Wright 2003; Morgan 2003; Little 2003). These consequences, known as radiation induced biological bystander effects (RIBBE), may contribute significantly to the effectiveness of radiotherapy by sterilising malignant cells which have not been directly irradiated.
In recent years, targeted radionuclide treatment of cancer has developed as a novel and advantageous approach to radiation therapy. The basic idea is to deliver higher doses of radiation to tumor than to normal cells by means of radionuclides chemically conjugated to tumor-seeking targeting agents such as meta-iodobenzylguanidine (MIBG). By virtue of its structural similarity to noradrenaline (Wieland et al 1980), MIBG is selectively accumulated via the noradrenaline transporter (NAT) (Jacques et al 1984) which is expressed on the surfaces of cells comprising tumors of neural crest origin, for example neuroblastoma and phaeo-chromocytoma. Radiolabelled forms of this drug are used for scinti-graphic assessment and treatment of such tumors (Simpson and Gaze 1998; Klingebiel et al 1998; Hoefnagel 1999; Rose et al 2003). NAT expression is predictive for MIBG uptake capacity (Mairs et al 1994) and quantification of NAT mRNA could be used for the selection of patients for MIBG therapy (Carlin et al 2003).
It may be possible to compensate for heterogeneity of uptake of radio-pharmaceutical, resulting in underdosing of some tumor regions, by the selection of radionuclides whose decay particles have long path lengths, enabling cross-fire irradiation of surrounding untargeted cells. However, even with long range radionuclides, because their emissions are of low LET, sub-populations of tumor cells will receive less than a sterilizing dose. Then again, emerging evidence suggests that radioactive emission from targeted cells is not the only bystander effect operating in radionuclide treatment of cancer. It is becoming clear that radiation-induced biological bystander effects (RIBBE) deriving from the cellular processing of the physical radiation insult, which need not interact directly with DNA, may play an important part in the overall efficacy of radionuclide targeting.
RIBBE predominate at low radiation dose and low dose rate (Carlsson et al 2003) both of which are features of targeted radionuclide treatment of cancer. Therefore bystander effects induced by radiopharmaceuticals may play a disproportionate role in the efficacy, and understanding their nature should enable the refinement of radiotherapy. Most investigations of bystander effects have involved external beam γ-irradiators and microbeams. However, in recent years important studies have been conducted of RIBBE after intracellular concentration of radiolabelled drugs. Bishayee et al (2001) prepared clusters composed of unlabelled and [3H]thymidine-labelled cells. The resulting death of unlabelled cells was considered to be a consequence of transfer of toxic factors from cells which had incorporated [3H]thymidine in their DNA rather than crossfire irradiation because 3H beta-decay particles have a path length which is too short to allow direct bombarment of regions adjacent to targeted cells. Survival of unlabelled cells was increased by treatment with dimethyl sulphoxide and lindane, suggesting the involvement of free radicals and gap junctional communication respectively. In a similar study, Xue et al (2002) mixed colon carcinoma cells, unlabelled or incubated with the thymidine analogue [125I]IUDR, and used these mixtures to form subcutaneous tumors in athymic mice. Again, inhibition of tumor growth was attributed to RIBBE because the range of 125I Auger electrons is insufficient to interact directly with neighboring cells.
Here we describe bystander responses following the uptake of radio-haloanalogues of MIBG by NAT gene-transfected tumor cell lines. Our results provide further insight into the dependence of targeted radiotherapy on RIBBE and the influence of radiation quality.
II. TRANSFECTANT MOSAIC SPHEROIDS: A NEW MODEL FOR EVALUATION OF TUMOR CELL KILLING IN TARGETED RADIOTHERAPY AND GENE THERAPY
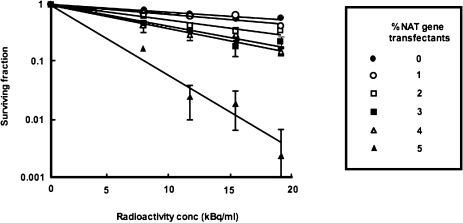
An attractive characteristic of therapeutic strategies which combine gene manipulation with the administration of radiopharmaceuticals is cross-fire irradiation of non-transfected cells. We assessed the usefulness of this effect using our recently developed mosaic spheroid model (Boyd and Mairs 2004) composed of mixtures of transfected and untransfected cells (Boyd et al 2002) (Figure 1). This allowed our evaluation of the minimum percentage of transfection required to achieve cure of different sizes of metastases. For example: modest radioactivity concentrations (20 kBq/ml) of [211At]MABG induced the complete sterilisation of 250μm diameter mosaic spheroids composed of only 5% NAT gene trans-fectants (Figure 2). Because the path length of 211At α-particles is only 55 to 70 μm, cross-fire irradiation from targeted to untargeted cells would be considerably less extensive than that from a β-emitter such as 131I. Therefore this observation suggests that bystander effects, over and above cross-fire irradiation, are operating in α-particle targeted therapy.
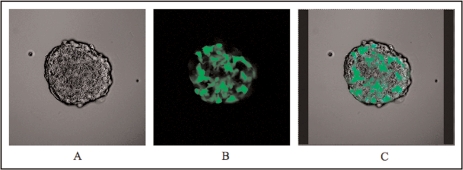
FIGURE 1.
Fluorescent confocal microscopic images of optical sections through a transfected mosaic spheroid composed of 50% NAT-expressing cells and 50% GFP-expressing cells. (A) phase contrast image of an equatorial optical section showing all cells present within the spheroid; (B) GFP-expressing cells only; (C) merged image showing GFP-labelled cells superimposed upon the phase contrast image.
FIGURE 2.
Toxcity, measured by clonogenic assay, of [211At]MABG to UVW spheroids containing various percentages of NAT gene-transfected cells. Means and standard deviations of triplicate determinations.
These studies indicate the potential for bystander-mediated cell kill and improved clinical efficacy of tumor targeting when only a small proportion of the tumor mass expresses the radiotherapeutic molecular target, in this case, introduced via gene modification. Moreover, RIBBE could compensate for the low levels of gene delivery currently achievable in vivo in cancer gene therapy strategies when married with targeted radionuclide therapy. Rational selection of radiohaloconjugates of MIBG will enable the enhancement of cross-fire kill to maximize neuroblastoma cell kill.
III. MEDIA TRANSFER METHOD FOR ASSESSMENT OF RIBBE
Our procedure for determining bystander responses in vitro employs an adaptation of the media transfer system developed by Mothersill and Seymour (1997) to compare the induction of bystander effects by external beam γ-radiation with those generated by MIBG labelled with radio-nuclides emitting β-particles, α-particles, or Auger electrons (Boyd et al 2006). The cells used in these experiments were transfected with the NAT gene to facilitate the active uptake of radiolabeled MIBG. Similarly treated control cells were untransfected and hence incapable of concentration of the radiopharmaceutical. This allowed our determination of the requirement for intracellular accumulation of radionuclide for induction of RIBBE.
For investigation of RIBBE following γ-irradiation, donor cells were directly irradiated and their medium was transferred to recipient cells which were not directly irradiated. Separate cultures (termed “direct”) were directly irradiated and their medium was not removed. Consequently the direct cells experienced the physical and biological effects of the radiation treatment. For the evaluation of RIBBE in radio-pharmaceutical-treated cells, it was necessary to control for killing of recipient cells due to the transfer of radiopharmaceutical effluxed from donor cells. To cultures, designated activity controls, was administered radiopharmaceutical activity equivalent to that which had leaked from donor cultures and would have been transferred to recipient cells. 24 h after these manipulations, cell kill was determined by clonogenic assay (Figure 3).
FIGURE 3.
Media transfer method for assessing bystander effect after radiopharmaceutical treatment of cultured cells.
IV. RIBBE INDUCED BY γ-IRRADIATION AND TARGETED RADIOPHAR-MACEUTICALS
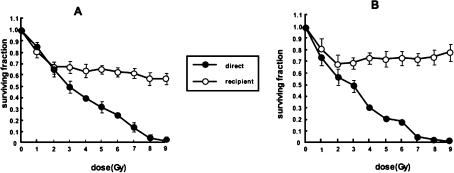
We observed that exposure of two human tumor cell lines—UVW glioma and EJ138 bladder carcinoma—to media derived from external beam irradiated cells produced a dose-dependent reduction in survival fraction in the dose range 0 to 2 Gy, followed by a plateau in clonogenic cell kill at levels greater than 2 Gy (Figure 4). Similarly, other reports of media transfer experiments, following treatment with γ-rays or soft x-rays, have indicated that the dose-response in bystander cells reached a plateau at low doses (Mothersill and Seymour 1997; Seymour and Mothersill 2000; Belyakov et al 2001).
FIGURE 4.
Survival of UVW/NAT cells (A) and EJ138/NAT cells (B) following treatment with γ-radiation (direct) or medium from irradiated cells (recipient). Means and standard deviations of six experiments performed in triplicate.
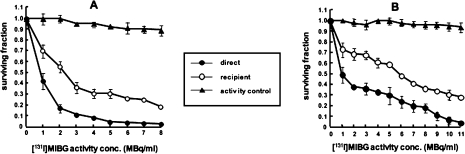
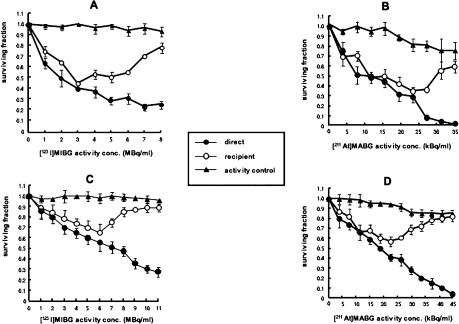
In contrast, no such plateau with respect to clonogenic cell kill was evident in recipients of medium from NAT-expressing cells incubated with [131I]MIBG (Figure 5). The potency of RIBBE produced by NAT-expressing cells after treatment with Auger electron emitting [123I]MIBG or α-particle emitting [211At]MABG, increased with activity up to levels which resulted in a direct kill of 35 to 45% (EJ cells) or 60 to 70% (UVW cells) of clonogens. At higher activity concentrations of [123I]MIBG or [211At]MABG, RIBBE became progressively weaker (Figure 6). The U-shaped survival curves associated with intracellularly concentrated high-LET emitters suggest that, after intracellular bombardment, the RIBBE-generating apparatus is inhibited above a threshold radiation dose. The RIBBE elicited by the pre-nadir activity range of these high LET targeted radionuclides resulted in a magnitude of cell kill similar to that caused by direct irradiation, indicating that bystander effects may be significant contributors to the cytotoxicity of [123I]MIBG and [211At]MABG at low activity concentrations. It will be important to the development of tumor targeting by [125I]- and [211At]-labeled compounds to determine differences in the nature of toxins generated at low (pre-nadir) and high (post-nadir) radioactivity concentrations.
FIGURE 5.
Survival of UVW/NAT cells (A) and EJ138/NAT cells (B) following treatment with [131I]MIBG (direct) or medium from [131I]MIBG-treated cells (recipient). Cultures designated activity control received radiopharmaceutical activity equivalent to that which had leaked from donor cultures and would have been transferred to recipient cells. Means and standard deviations of six experiments performed in triplicate.
FIGURE 6.
Survival of UVW/NAT cells [(A) and (B)] and EJ138/NAT cells [(C) and (D)] following treatment with [123I]MIBG and [211At]MABG (direct) respectively or medium from radiopharma-ceutical-treated cells (recipient). Cultures designated activity control received radiopharmaceutical activity equivalent to that which had leaked from donor cultures and would have been transferred to recipient cells. Means and standard deviations of six experiments performed in triplicate.
Significantly, neither direct nor indirect kill was observed at any activity concentration in cultures of cells which did not express the NAT and were incapable of active uptake of MIBG. Thus, these RIBBE effects were not related to decays of unbound radiopharmaceutical present in the media. These findings suggest that potent toxins are generated specifically by cells which concentrate targeted radionuclides. Furthermore, a comparison of the dose dependence of these effects with those observed after exposure to γ-rays suggests that RIBBE effects from targeted radio-therapeutics may be distinct from those elicited by conventional external beam radiotherapy.
V. CONCLUSION
The successful application of targeted radionuclide cancer therapy is critically dependent on the delivery of cytotoxic doses of radiation to the vast majority of the malignant cell population. Achieving this goal can be confounded by multiple factors, including variations in target molecule expression and tumor hemodynamic parameters, that can lead to heterogeneity in radiopharmaceutical delivery, retention or binding. However, it has been widely appreciated that cells not accumulating the labelled molecule can be killed as a result of being hit by radiation emitted from neighboring cells. Consideration of this process, known as the (physical) bystander effect, has played an important role in the design of radiotherapeutic strategies, for example, the selection of long range β-particle emitters in situations where heterogeneous radiopharmaceutical delivery is anticipated.
A second type of bystander effect that could have important implications for targeted radionuclide therapy is the radiation induced biological bystander effect (RIBBE), which results in the killing of cells not directly exposed to radiation. The mechanisms involved are as yet undefined. However, studies using γ-ray and α-particle beams have provided some insight into possible factors. These include oxidative stress leading to increased radical formation (Lehnert and Goodwin 1997; Narayanan et al 1997) nitric oxide release (Matsumoto et al 2001; Shao et al 2002) cytokine release (Iyer and Lehnert 2000) and gap junctional intracellular communication (Azzam et al 2001). RIBBE resultant from targeted radionuclides has largely been unexplored and the effects of radiation quality remain unknown (Mairs and Boyd 2005).
Elucidation of the pathways involved in RIBBE generation by radionuclides could indicate ways of manipulating RIBBE production to reduce toxicity to normal tissues which are inadvertently irradiated during the course of a targeted radiotherapy regime. Careful choice of radionuclides and dose administered in clinical scenarios for targeted radionuclide therapy of tumors which naturally accumulate targeted radionuclides or have been genetically manipulated to do so, will allow factors such as inefficient gene transfer and heterogeneous uptake to be compensated for, thus optimising the cell kill potential of this therapeutic scheme.
Whatever the mechanism, RIBBE could be important not only in relation to radiation protection and safety but also with respect to the therapeutic use of ionizing radiation. Exploitation of RIBBE could be especially relevant to the efficacy of targeted radiotherapy because this treatment is limited by heterogeneous uptake of radionuclides by tumors. Freely diffusible toxic bystander signals could overcome the inefficiency of tumor control due to non-uniform distribution of radiation dose.
We seek now to investigate the nature of RIBBE signals generated by radiopharmaceuticals localized to different sub-cellular regions. The efficiency of this mode of kill in tumor and normal cells and its possible dependence on genetic background and tumor microenvironment must also be assessed. From a practical perspective, the identification of RIBBE factors will stimulate the design of strategies to maximize damage to tumor cells while minimizing damage to normal cells.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Department of Health, The Clerk Maxwell Cancer Research Trust, Cancer Research UK, the Neuroblastoma Society, the British Urological Foundation, the Royal College of Physicians and Surgeons of Glasgow (Ian Sunter Charitable Trust Research Fellowship), the National Institutes of Health (Grant CA42324) and the Pediatric Brain Tumor Foundation.
The authors wish to thank Drs. Ganesan Vaidyanathan, Department of Radiology, Duke University Medical Centre, Durham, North Carolina, USA and Sally L. Pimlott, Radionuclide Dispensary, Western Infirmary, Glasgow, UK, for synthesis of the radiopharmaceuticals used in these experiments.
REFERENCES
- Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap-junction mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to non-irradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov OV, Malcolmson AM, Folkard M, Prise KM, Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Brit J Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiat Res. 2001;155:335–344. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M, Mairs SC, Stevenson K, Livingstone A, McCluskey AG, Ross SC, Mairs RJ. Transfectant mosaic spheroids: a new model for the evaluation of bystander effects in experimental gene therapy. J Gene Medicine. 2002;4:567–576. doi: 10.1002/jgm.293. [DOI] [PubMed] [Google Scholar]
- Boyd M, Mairs RJ. Tumour spheroids. In: Freshney RI, editor. The culture of animal cells. 5th edition. New York: Alan R. Liss; 2004. pp. 281–298. [Google Scholar]
- Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, Mairs RJ. Radiation induced biological bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with a, ß and Auger electron emitting radionuclides. J Nucl Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- Carlin S, Mairs RJ, McCluskey AG, Tweddle DA, Sprigg A, Estlin C, Board J, George RE, Ellershaw C, Pearson ADJ, Lunec J, Montaldo PG, Ponzoni M, van den Brug MD, Tytgat GAM, Caron HN. Development of a real-time polymerase chain reaction assay for prediction of the uptake of [131I]meta-iodobenzylguanidine by neuroblastoma tumours. Clin Cancer Res. 2003;9:3338–3344. [PubMed] [Google Scholar]
- Carlsson J, Aronsson EF, Hietala S-O, Stigbrand T, Tennvall J. Tumour therapy with radionuclides: Assessment of progress and problems. Radiother Oncol. 2003;66:107–117. doi: 10.1016/s0167-8140(02)00374-2. [DOI] [PubMed] [Google Scholar]
- Hoefnagel CA. Nuclear medicine therapy of neuroblastoma. Quart J Nucl Med. 1999;43:336–343. [PubMed] [Google Scholar]
- Iyer R, Lehnert BE. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- Jacques S, Jr, Tobes MC, Sisson JC, Baker JA, Wieland DM. Comparison of the sodium dependence of uptake of meta-iodo-benzylguanidine and norephrine into cultured bovine adrenomedullary cells. Mol Pharmacol. 1984;26:539–546. [PubMed] [Google Scholar]
- Klingebiel T, Bader P, Bares R, Beck J, Hero B, Jurgens H, Lang P, Niethammer D, Rath B, Handgretinger R. Treatment of neuroblastoma stage 4 with [131I]meta-iodobenzylguani-dine, high dose chemotherapy and immunotherap. A pilot study. Eur J Cancer. 1998;34:1398–1402. doi: 10.1016/s0959-8049(98)00130-0. [DOI] [PubMed] [Google Scholar]
- Lehnert BE, Goodwin EH. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- Little JB. Genomic instability and bystander effects: a historical perspective. Oncogene. 2003;22:6978–6987. doi: 10.1038/sj.onc.1206988. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Wright EG. Radiation-induced genomic instability and bystander effects: Related inflammatory-type responses to radiation-induced stress and injury? A review. Int J Radiat Biol. 2003;79:15–25. [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Protect Dosimetry. 2002;99:169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- Mairs RJ, Livingstone A, Gaze MN, Wheldon TE, Barrett A. Prediction of accumulation of 131I-labelled meta-iodobenzylguanidine in neuroblastoma cell lines by means of reverse transcription and polymerase chain reaction. Brit J Cancer. 1994;70:97–101. doi: 10.1038/bjc.1994.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairs RJ, Boyd M. Low dose radiation and bystander effects. Int J Radiat Biol. 2005;81:259–262. doi: 10.1080/09553000500091907. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T, Kano E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat Res. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionising radiation? Oncogene. 2003;22:7094–7099. doi: 10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation induced bystander effects: Past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects-implications for cancer. Nat Rev Cancer. 2004;4:158–64. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Medium from irradiated human epithelial cells but not human fibroblasts reduced clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Relative contribution and targeted cell killing to the low-dose region of the radiation dose-response curve. Rad Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rose B, Matthay KK, Price D, Huberty J, Klencke B, Norton JA, Fitzgerald PA. High-dose 131I-Metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma. Cancer. 2003;98:239–248. doi: 10.1002/cncr.11518. [DOI] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int J Radiat Biol. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- Simpson JK, Gaze MN. Current management of neuroblastoma. Oncologist. 1998;3:253–262. [PubMed] [Google Scholar]
- Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Bierwaltes WH. Radiolabelled adrenergic neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med. 1980;21:349–353. [PubMed] [Google Scholar]
- Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabelled tumour cells in vivo. Proc Natl Acad Sci USA. 2002;99:13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]