Abstract
The induction of “bystander effects” i.e. effects in cells which have not received an ionizing radiation track, is now accepted but the mechanisms are not completely clear. Bystander effects following high and low LET radiation exposure are accepted but mechanisms are still not understood. There is some evidence for a physical component to the signal. This paper tests the hypothesis that bioelectric or biomagnetic phenomena are involved. Human immortalized skin keratinocytes and primary explants of mouse bladder and fish skin, were exposed directly to ionizing radiation or treated in a variety of bystander protocols. Exposure of cells was conducted by shielding one group of flasks using lead, to reduce the dose below the threshold of 2mGy 60Cobalt gamma rays established for the bystander effect. The endpoint for the bystander effect in the reporter system used was reduction in cloning efficiency (RCE). The magnitude of the RCE was similar in shielded and unshielded flasks. When cells were placed in a Faraday cage the magnitude of the RCE was less but not eliminated. The results suggest that liquid media or cell-cell contact transmission of bystander factors may be only part of the bystander mechanism. Bioelectric or bio magnetic fields may have a role to play. To test this further, cells were placed in a Magnetic Resonance Imaging (MRI) machine for 10min using a typical head scan protocol. This treatment also induced a bystander response. Apart from the obvious clinical relevance, the MRI results further suggest that bystander effects may be produced by non-ionizing exposures. It is concluded that bioelectric or magnetic effects may be involved in producing bystander signaling cascades commonly seen following ionizing radiation exposure.
Keywords: Radiation-induced bystander effect, bioelectric effects, explant cultures, fish cells, murine bladder
INTRODUCTION
The whole spectrum of effects associated with direct exposure to low doses of ionizing radiation can be observed in so-called “bystander cells”, which are in receipt of media from exposed cells (medium transfer protocols), or which are in the same cell population but do not receive a direct hit (microbeam or low particle fluence protocols). For state-of-the-art reviews see (Little and Morgan, 2003, Lorimore and Wright, 2003, Morgan, 2003, Mothersill and Seymour, 2004). While much is known about the phenomenology and increasingly, about the signal transduction in recipient cells, very little is understood about the nature of the signal itself and how it is produced or induced by radiation (Mothersill et al. 2000, Iyer and Lehnert, 2002, Lyng et al, 2002a, Geraschenko and Howell, 2003). Oxidative stress is a longterm consequence of low dose radiation exposure (Clutton et al, 1996, Limoli et al 1998, Rugo and Schiestl, 2004) and this has led to consideration of long-lived radicals being involved (Spitz et al, 2004, Matsumoto and Ohnishi, 2004) but media harvest protocols suggest that the signal or the property of medium leading to bystander effects is very long-lived (hours – days), which is too long for radicals to persist. Other data in this and related fields suggest that mitochondrial and or membrane depolarization events are critical elements involved in the induction and or expression of signaling including bystander signaling (Murphy et al, 2005, Nelson and Melendez, 2004). There is evidence that ion gated channels open and allow influx of calcium ions, which initiate apoptosis (Lyng et al, 2000, 2002b). Mitochondrial membrane depolarization also occurs (Maguire et al, 2005). These events can be prevented by antioxidants and by inhibitors of monoamine oxidases (Emerit et al, 1997, Seymour et al, 2003, Konopacka and Rzeszowska-Wolny, 2006) or by inhibition of monoamine binding to irradiated cells (Poon et al, accepted for publication). Melanin is also known to protect against bystander effects and can prevent activity even when added to bystander cells rather than irradiated cells (Mosse et al 2006). Melanin is known to act as a radioprotector by absorbing radiative energy of all wavelengths, leading Mosse et al (2006) to speculate that perhaps the bystander signaling mechanism has a physical component. A common thread in all these observations is that electrophysiological activity is associated with bystander effects. The aim of this research is to test the hypothesis that the bioelectric mechanisms have a role to play in the initiation of bystander effects by ionizing radiation.
METHODS
Clonogenic cell line
HPV-G cells are adherent epithelial cells derived originally from a human foreskin primary culture and immortalised by the HPV virus (Pirisi et al, 1988) They were obtained as a gift from Prof J DiPaolo (NIH, Bethesda) and have been used in our laboratory as a reporter system for bystander signal production in a wide range of experiments (e.g. Mothersill et al 2001, 2005). The cell line was originally grown in DMEM:F12 (1:1) obtained from Invitrogen, Burlington, Canada but for the explant experiments, cells which had been pre-adapted for at least 3 months into RPMI medium used for explant culture (see below) were used. After three months the growth and cloning efficiency of the reporter cells was identical to the parent line. Both media were supplemented with 10% Foetal bovine serum, 1microgram /ml hydrocortisone (Sigma, Mississauga, Canada), 5ml penicillin : streptomycin solution and 5ml L-Glutamine solution. Hepes buffer (15mM final conc.) was added to help maintain the pH at 7.4 in ambient air atmosphere. Except where indicated, all reagents were obtained from Invitrogen. The line was maintained in T75 flasks (NUNC Inc. Uden, Netherlands). The cells are non-tumourigenic and have about 30% of the normal keratinocyte wild-type p53 expression, the rest is suppressed by the HPV-virus E6 protein (Cooper et al, 2003) and have a normal epithelial pattern of cobblestone density inhibited cell growth. They are used because when exposed to autologous medium harvested from irradiated cells, they give a stable bystander effect of approximately 40% reduction in plating efficiency over a very wide range of doses and exposure conditions (Seymour and Mothersill, 2000). This allows comparison of bystander inducing signal strengths even when the HPV-G cells are exposed to signals from other cell lines or from explants.
Clonogenic assay for bystander activity using HPV-G cells as reporters:
Flasks which were 85–90% confluent and that had received a medium change the previous day were chosen. Cells were removed from the flask using 0.25 %w/v trypsin/ 1mM EDTA solution (1:1). When the cells had detached they were resuspended in medium, and the cell number per ml determined. Appropriate cell numbers were plated for survival using the clonogenic assay technique of Puck and Marcus (1956). Flasks destined to donate medium were plated with cell numbers in the region of 2×105 per 5ml medium (40,000 cells per ml) in T24, 40ml volume flasks (NUNC, Uden, Netherlands). Medium was harvested 1hour post irradiation, which took place 6 hours after plating. The harvested medium was transferred to cultures containing cloning densities of cells set up at the same time as the donors. Controls for medium only and actual irradiation effects were included in each experiment. Controls for transfer of unirradiated medium from densely seeded cultures to cultures seeded at cloning densities were also always included. Cultures were incubated in a humidified 37°C incubator in an atmosphere of 5% CO2 in air.
Medium transfer
The technique used has been described in detail in (Seymour and Mothersill 2000). Briefly, medium was poured off donor flasks (containing explants or HPV-G cells). This was filtered through a 0.22 μ filter. This was to ensure that no cells could still be present in the transferred medium. Intact cells were not present in the filtered supernatants (as detected by examination of aliquots of medium under the microscope). Culture medium was then removed from the flasks designated to receive irradiated conditioned medium and the filtrate was immediately added to these recipient flasks. A medium change of unirradiated but similarly filtered medium from unirradiated donor flasks containing explants or seeded at the donor density of approx. 200,000 cells per flask was given to controls at the same time. Standard plating efficiency controls were also set up. There was never a significant difference between these two controls. Standard clonogenic survival points following direct irradiation were also always included, with and without a medium change at the appropriate time. No effect of changing the medium alone was found.
Tissue explant technique
Explants of murine bladder and rainbow trout skin were established as described previously (Mothersill et al, 1995, 2001), any changes to the protocol are incorporated in the brief description below. The C57Bl6/C129 mice were sacrificed by cervical dislocation, bladders were removed, pooled and placed in complete growth medium. They were dissected into four equal sized pieces per bladder and incubated for 30 min at 37°C in 0.25% w/v trypsin (Invitrogen, Burlington, Canada), containing a solution of 10mg/ml collagenase, type IV (Sigma, Mississauga, Canada). At the end of the incubation time the explants were washed in growth medium and plated as single explants in the centre of 24cm2 growth area, 40ml volume flasks (NUNC, Uden, Denmark) in 2 ml growth medium. Flasks were left undisturbed for 24hr at 37°C in an atmosphere of 5%CO2 in air. Rainbow trout skin samples were treated similarly but the samples were incubated at 19°C without CO2 and were exposed in vitro to 0.5Gy gamma irradiation 24 hrs after plating. Where multiwells were used, donor cell numbers (200,000 cells) or single explants were seeded on the multiwell insert in wells containing 2ml culture medium and irradiated 6hr later (cells) or 24hr later (explants). One hr after irradiation, the insert was removed and 100 reporter cells were seeded into the medium in the well. For direct effect controls, clonogenic numbers of reporters were seeded in multiwells before irradiation. These were left for 9 days to form colonies.
Irradiation of tissue and cell cultures
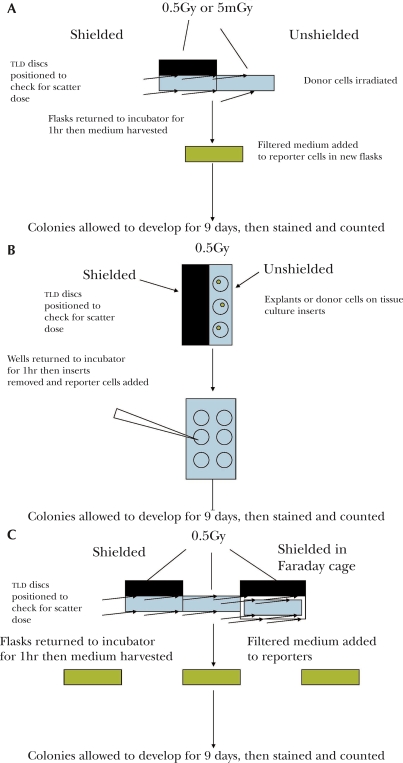
Where indicated, cultures were irradiated at room temperature using a standard radiotherapy cobalt 60 teletherapy unit located and maintained at the Juravinski Cancer Centre in Hamilton. The flask to source distance (FSD) routinely used for these experiments was 80cms and the field size was 30×30cms. The cobalt beam is u-shaped with very small fall off at the edges but to be sure of dose homogeneity, a rim of 5cms was left between the flasks and the edge of the field. Thermoluminescent discs (TLD's) were used to ensure the doses were accurate in all locations within the beam. The beam is also routinely calibrated by Physics staff at the Centre. Irradiation commenced 6 hrs after plating for cell lines and 24hr after plating for tissue explants. The dose rate at 80cms FSD was approximately 1.8Gy/min during these experiments. Where very low doses of radiation were used, flasks were placed on the floor under the beam, The FSD was then 200cms and the dose rate was approximately 0.3Gy/min. Flasks were placed side by side when the shielding experiments were done, with no space between the shielded and the unshielded flasks (see Figure 1a). Shielding was done using lead blocks which were 5cms thick. TLD discs were placed above the lead, between the lead and the flask/multiwell and under the flask. TLD's were spaced out over the flasks particularly at the edge of the shielding lead to ensure that shielding of all parts of the flask was effective. Experiments were also repeated with and without shielding at a dose of 5mGy, which is just above the threshold known to induce a bystander effect. In the case of multiwells, 6 well plates were used and three were shielded and the other three were not (see Figure 1b). The explant multiwell experiments were done by irradiating the shielded or unshielded explant, which was on a tissue culture insert in the well. After one hour the explant was removed and reporter cells were introduced into the well.
FIGURE 1.
Flow diagrams showing the protocol for the irradiation of flasks (1a), explants/multiwells (1b) and the second series of experiments using a Faraday cage (1c).
Faraday cage construction
Faraday cage was constructed from copper clad (0.034mm thick) fiberglass epoxy resin (unetched) circuit board (2mm thick). The dimensions of the cage were 15×8×10cms. To maintain the shield, the edges of the cage were soldered together using standard resin core solder, and the cage was electrically grounded. In a similarly constructed box (Del Seppia, et al, 2002), measurements with a fluxgate magnetometer indicate that there is essentially no magnetic field attenuation at frequencies below 300Hz, whereas the electric field is shielded effectively. The experimental protocol for irradiating cells in this cage is shown on Figure 1c.
Scatter dose experiments
Because the threshold for the bystander effect is very low in our cell line, scatter dose was measured by placing TLD discs at various points inside and outside the Faraday cage. Experiments were also set up using 5mGy as the delivered dose (at 200 cm source to flask distance) before shielding reduction and TLD discs were used to quantify the scatter dose in shielded and unshielded situations.
MRI exposure
Cultures of HPV-G cells were set up using an identical protocol to that developed for ionizing radiation exposures. Cells were subjected to typical static and time-varying magnetic fields that would be routinely generated by a standard magnetic resonance imaging (MRI) protocol using a 1.5Tesla (T) Siemens Symphony (Siemens, Erlangen, Germany). The cells were placed inside the Siemens head coil and a 10 minute protocol was applied. Coronal slices were selected from the localizer [:09] (9 seconds) and the following sequences were applied:
T1 weighted Turbo Spin-Echo (35cm FOV, 4mm slice, TR/TE = 500/13ms, 512×256 matrix size, 166 degree flip angle) [2:54]
TrueFISP (35cm FOV, 6mm slice, TR/TE = 4.4/2.2ms, 256×256 matrix size, 70 degree flip) [:30]
T2 weighted Gradient Recalled Echo (22cm FOV, 5mm slice, TR/TE = 430/9.5ms, 256×192 matrix size, 20 degree flip) [2:16]
T2 Weighted Turbo Spin-Echo (24cm FOV, 6mm slice, TR/TE = 3700/89ms, 512×256 matrix size, 166 degree flip angle) [4:32]
Sham controls were placed in the MRI machine for the same duration but the machine was not turned on.
Statistical Analysis
Data are presented as mean ± standard error for at least three independent experiments with at least three replicate flasks per experiment and significance was determined using the student t-test.
RESULTS
Effect of shielding using lead on gamma-ray induced reduction in cloning efficiency of bystander cells
Table 1 shows the data obtained for HPV-G cells seeded in flasks or multiwells at medium donor cell densities of 200,000 cells per 5ml medium for determination of bystander effects, or at clonogenic cell densities (500 cells) for estimation of the direct effects of the 0.5Gy dose. Flasks were placed side by side with the plastic in contact, under the cobalt beam but one set were shielded using lead blocks. This reduced the dose to less than 1mGy (measured using TLD discs), which is below the dose known to induce bystander signals in this system (Liu, et al, 2006). When multi-wells were used, three wells of each six well plate were shielded, the other three were not. The cultures destined for measurement of direct effects were incubated without further manipulation. Medium was harvested after one hr from the donor cultures, filtered and added to unirradiated reporter cells seeded and cloning densities in T24 flasks using the protocol described in the methods. The results in table 1 show that while the direct dose effect was prevented by the shielding, the bystander effect was the same in shielded and unshielded flasks. The same result was obtained using multiwells. These experiments were repeated using a 5mGy dose with and without shielding where the scatter dose to the shielded flasks was below the limit for detection of dose using TLD discs (<0.5mGy). This is further confirmation that the effects being measured were not due to scatter dose.
TABLE 1.
The effect of lead shielding during irradiation on the direct response to the dose or on the production of bystander signals by donor cells.
| Treatment | No shielding flasks | No shielding multiwells | Lead shielding flasks | Lead shielding multiwells |
|---|---|---|---|---|
| OGy direct irradiation | 100 (31.7±1.6) | 100 (24.3±1.7) | 103±3.2 | 99.2±4.5 |
| 0.5Gy direct irradiation | 73.6±2.5 | 76.7±4.1 | 104.3±3.6 | 103±4.4 |
| 5Gy direct irradiation | 17.5±0.83 | 16.8±1.3 | 96.2±5.8 | 94.3±6.3 |
| OGy bystander medium | 100 (30.2±1.7) | 100 (23.2±1,4) | 101±4.7 | 105±6.7 |
| 0.5Gy bystander medium | 63.2±4.3 | 76.1±3.9 | 56.7±2.7 | 62.5±3.2 |
| 5Gy bystander medium | 60.8±3.7 | 71.0±4.2 | 59.4±3.3 | 66.7±4.0 |
For direct irradiation, clonogenic cell numbers were irradiated and left to grow to form colonies. For bystander points, media samples were harvested from donor cells, filtered and placed on clonogenic cell numbers of cells pre-seeded 6 hr earlier in T25 flasks. Data are the % of control plating efficiencytSEM, the absolute plating efficiency of the controls is shown in brackets. N=9
Experiments using explants:
To look at this effect in a tissue equivalent system, similar experiments were set up using explants of fish skin and mouse bladder, both known from previous experiments to give significant bystander effects using the HPV-G reporter system. In this case the reporter cells were added to the multiwell 1hr after irradiation at which time the explant which was on a tissue culture insert, was removed. The results are shown on table 2 and again confirm that shielding of explants from three wells of a multiwell plate did not prevent the bystander effect from occurring in reporter cells added 1hr after irradiation of the explant had taken place. The inclusion of control wells containing medium but no explant ever, confirm that this result is not due to the production of toxic agents in irradiated medium but that it requires pre and/or post irradiation conditioning of the medium by explants.
TABLE 2.
Repeat of experiments in table 1 using explant cultures offish skin and mouse bladder.
| Treatment | Explant exposed without shielding | Lead shielded explant |
|---|---|---|
| Control no explant OGy to medium | 100 (24.3±1.1) | 100 (21.3±0.9 |
| OGy to fish skin | 105±3.6 | 97.6±3.8 |
| OGy to mouse bladder | 103±5.8 | 97.5±3.5 |
| Control no explant 0.5Gy to medium | 103±6.3 | 101±5.4 |
| 0.5Gy to fish skin | 56.9±2.2** | 66.7± 2.5** |
| 0.5Gy to mouse bladder | 23.2±0.7*** | 19.1±0.89*** |
| Control no explant 5Gy to medium | 99.3±5.2 | 103±3.8 |
| 5Gy to fish skin | 53.1±1.9** | 53.1±3.1** |
| 5Gy to mouse bladder | 22.1±3.6*** | 16.4±1.2*** |
In each case explants were plated singly on multiwell inserts and irradiated after 24 hrs. lhr post exposure, the inserts were discarded and 100 reporter cells added to the multiwells. Controls were irradiated multiwells with inserts and medium but no cells added until lhr post irradiation.
p<0.01,
p<0.001. n=9
Experiments using a Faraday cage
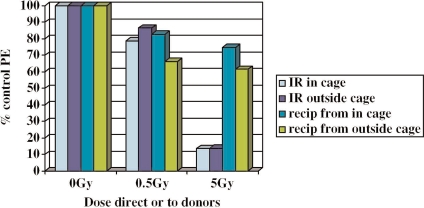
The above results are not compatible with the bystander effect being purely due to transmissible or diffusible factors because cells “next door” to irradiated cells produce bystander effects even though the ionizing radiation (scatter) dose has been reduced to below the threshold for inducing bystander effects. This could suggest a physical component to the bystander effect. To test whether bioelectric fields could be involved, a Faraday cage was constructed as described in the methods section. Faraday cages eliminate the electric field but do not attenuate the magnetic field. Two protocols were used, in protocol 1 cell cultures were set up with donor (200,000) and clonogenic (500) cell numbers and irradiated 6hrs later either inside or outside of the cage. Culture flasks were only in the cage during the actual ionizing radiation exposure and were returned to the incubator immediately afterwards. The results shown on Figure 2 suggest that irradiation inside the cage did not fully ablate the bystander effect but it did reduce it. To exclude the possibility that bioelectric fields generated by the beam inside the cage, were causing the effect, further experiments were done using the protocol outlined in Figure 1c where cultures were irradiated with and without shielding within and without the Faraday cage. TLD discs were used to check that the ionizing radiation dose was effectively reduced by the shielding. The results shown on Table 3 indicate that irradiation in the cage with shielding did reduce the bystander effect but again a significant bystander effect could be detected. Irradiation in the cage had no effect on the response to direct irradiation. When shielding was used the surviving fraction in the directly exposed group was the same as the controls, indicating again that shielding was effective in attenuating the radiation dose.
FIGURE 2.
% of reporter cell control plating efficiency when cells are directly irradiated inside or outside a Faraday cage, or when medium is harvested from cells exposed inside or outside the cage. “IR” = ionizing radiation, “Recip” = recipients of irradiated cell conditioned medium. 0.5Gy irradiated or recipient cells were significantly different from unexposed cells (p<0.05), but were not significantly different from each other. 5.0Gy irradiated or recipient cells were significantly (p<0.01) different from unexposed controls. The Faraday cage did not significantly affect the plating efficiency of these groups.
TABLE 3.
Effect of using a Faraday cage with shielding during irradiation of cells on the direct and bystander effects.
| Treatment | % control plating efficiency |
|---|---|
| Control | 100 (23.5±1.6) |
| 0.5 Gy Direct no shield | 78.3±6.1* |
| 0.5 Gy Direct shielded | 97.2±6.7 |
| 0.5 Gy Direct shielded and in Faraday cage | 98.0±5.4 |
| Control bystander | 100 (24.8±1.3) |
| 0.5 Gy bystander no shield | 60.7±4.4* |
| 0.5 Gy Bystander shielded | 63.1±4.1* |
| 0.5 Gy Bystander shielded and in Faraday cage | 75.6±4.3* |
Flasks were irradiated to 0.5Gy see Fig 2 for set up design. Flasks were removed immediately from the cage and replaced in the incubator. Harvest of bystander medium took place lhr later. Data are presented as mean plating efficiency of directly irradiated cells or unexposed reporter cells receiving medium from irradiated donors. Significance values are between controls and treated groups.
= p<0.05
Experiments with MRI
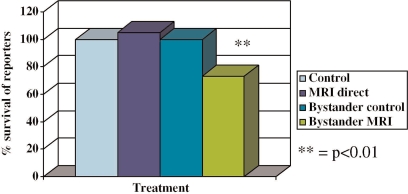
The data described above suggest that the bystander effect is not purely due to chemical factors going between cells, but that responses, which ultimately depend on chemical communication can be triggered in the absence of liquid mediated communication. They further suggest that ionizing radiation may be causing bioelectric effects which can affect cells shielded from the direct beam and that these associated effects rather than direct ionization may be implicated in the generation of downstream bystander responses. This means that non-ionising exposures such as those delivered during MRI, could generate bystander effects. To further test this hypothesis, some experiments were done using a clinical MRI exposure as described in the methods section. The results are presented in Figure 3. Clearly, significant bystander but not direct effects are produced following MRI exposure.
FIGURE 3.
% of reporter cell control plating efficiency following direct exposure to a clinical MRI sequence of exposures. Or following exposure to medium harvested from cells exposed to the MRI sequence. ** = p<0.01).
DISCUSSION
All the information world wide concerning bystander effects points to the existence of chemical entities which are produced as a consequence of the radiation exposure and this is not excluded by the findings presented in this paper. However, we show in this paper that bystander effects can occur in cells which are exposed to media harvested from cells exposed to the gamma ray beam while shielded. Our conclusion is that a physical property of the interaction between the radiation beam and the flaks/medium/cell complex, may be able to trigger donor cells to produce signals even if these donor cells are not directly exposed to ionizing radiation doses high enough to trigger the bystander effect. The data presented in this paper could support the idea that there may indeed be a physical component to the bystander mechanism. Exposure of human immortalized keratinocytes, mouse bladder explants and fish skin explants, in various experiments designed to dissociate the bioelectric field effect from the ionizing radiation effect, all show that when the ionizing component of the dose is reduced below the level necessary to trigger a bystander effect, one can still be detected, if the cells are beside cells being directly irradiated. Importantly, if cells are placed in an MRI field, they do produce a bystander effect but there is no direct effect of the MRI on cell survival. Our control flasks are sham irradiated so this excludes the possibility that disturbance, light or heat changes could explain the effect. The cultures seeded at low densities (approx 500 cells) for direct effect clonogenic assay measurements after a 0.5Gy dose or lower, never show the reduced plating efficiency. It is important to discuss here the lack of a direct effect of ionizing radiation in the shielded flasks. It might be expected that a bystander effect would have been induced under these circumstances. One probably is induced but since only 500 cells are present in the directly irradiated flasks compared to 200,000 cells in the medium donor flasks, it is not possible to generate sufficient bystander molecules into the medium. We have already demonstrated (2) that the bystander effect is dependent on having a high cell number to medium volume ratio. This means that a high cell number is necessary to produce the effect. Our previous conclusion concerning this point was that the radiation exposure triggered a process, the magnitude or strength of which could be quantified on a per cell basis (e.g. as a certain number of bystander factor molecules released per exposed cell). This assessment is probably still valid because clearly the donor cells still interact with medium and produce signals, but these signals do not require a liquid medium vehicle to be transmitted. Our hypothesis was that a candidate component of radiation which could be implicated is bioelectric fields associated with the cellular response to ionizing radiation exposure. Shielded cultures in proximity to the unshielded cultures would have been exposed to any such fields but not to the direct beam. Our attempts to actually measure a field associated with the radiation beam itself, failed, possibly because of the lack of sensitivity of the instrument used (limit of 20μT). Also it is likely that production of the biological EM field would require cells to be present. The fact that the Faraday cage reduced but did not completely eliminate the bystander effect, supports this idea, because the bioelectric field outside the Faraday cage due to the beam passing through the air could not have contributed but the beam inside the cage could have produced an associated field. Also the faraday cage does not affect any biomagnetic field. (Del Seppia et al 2002). The biomagnetic field appears to be important since exposing cells to an MRI clinical sequence for 10 minutes triggered a bystander effect. It is important to note here that Cobalt 60 does not have an associated electromagnetic field but this does exclude the possibility that when interacting with live cells, bioelectric phenomena occur. Membrane depolarization which does occur during irradiation (Maguire et al 2005), is one example of such a bioelectric effect. Thus while we have not direct evidence for production of a bioelectromagnetic field, there is theoretical and circumstantial evidence to support the possibility.
There is a wealth of evidence that bioelectric or magnetic fields can affect cells (Gandhi, 2002, Brodsky et al, 2003, Swanson and Kheifets, 2006, Yamaguchi et al, 2006), particularly by influencing membranes resulting in altered permeability to ions as a result of effects on the potential difference across the membrane (Jasti et al, 2001, Lohmann et al, 2003, Dini and Abbro, 2005). An association of growth factor production with bioelectric field exposure has also been reported (Aaron et al, 2004). Non-healing fractures can sometimes be helped by bioelectric or magnetic treatment and the mechanism is reported to involve TGFβ production due to the EM stimulation, (Bodamyali et al, 1998, Aaron et al, 2002). There is also a lot known about the ability of plastics to conduct EM waves and optical signals (Werneck and Barrientos, 1994, http://www.lumigen.com). Fibre optic technology relies on this and in the microplate reader industry, many precautions have to be taken to prevent optical conductance in microwell plates. Thus at one level these results suggesting that bystander effects may be transmitted via physical signals are not that surprising. We know that ion channels are important in the initiation of bystander responses (Lyng et al 2006) and have preliminary evidence that drugs which transduce electrochemical signaling via receptors associated with ion-gated membrane channels are involved in triggering signal production (Poon et al accepted for publication).
Whatever mechanisms are involved, this finding, if validated in other systems and most importantly in vivo, means that there is probably a physical component to the bystander effect which would mean that a signature biomarker could be defined, distinguishing low dose radiation exposures from other types of cellular events. This is a key issue for radiation protection and in the multiple stressor field where assigning causality to a single agent is usually impossible at low doses.
The data may also provide a biological mechanism for the poorly defined and often misunderstood plethora of effects attributed to bioelectric and biomagnetic fields (see reviews, Gandhi 2002, Brodsky et al, 2003). These effects have always been very refractive to epidemiological studies, and often cannot be repeated by different laboratories. This is probably because like bystander effects they result not in one biological effect but in many, and are probably best thought of as subtle facilitators of a wide range of events with a root cause of signal activation at the cell–tissue hierarchical level. Response to such signaling depends on the cellular status not on the “dose” of inducing agent.
To conclude, the data in this paper support a role for bioelectric fields associated with ionizing radiation, in the production of bystander effects and further provide evidence that MRI exposure can trigger bystander effects in cells in vitro.
ACKNOWLEDGMENTS
We acknowledge the Canada Research Chair Programme, the NSERC Industrial Research Chair programme and Discovery Grants Programme, CANDU Owner's group, Ontario Power Generation and Bruce Power for funding.
REFERENCES
- Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:30–37. doi: 10.1097/00003086-200402000-00006. Review. [DOI] [PubMed] [Google Scholar]
- Aaron RK, Wang S, Ciombor DM. Up-regulation of basal TGFbeta1 levels by EMF coincident with chondrogenesis - implications for skeletal repair and tissue engineering. J Orthop Res. 2002;20:233–240. doi: 10.1016/S0736-0266(01)00084-5. [DOI] [PubMed] [Google Scholar]
- Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, Abbott J, Blake DR, Stevens CR. Pulsed electromagnetic fields simultaneously induce osteogenesis and upreg-ulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Comm. 1998;250:458–461. doi: 10.1006/bbrc.1998.9243. [DOI] [PubMed] [Google Scholar]
- Brodsky LM, Habash RW, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part III: Risk analysis. Crit Rev Biomed Eng. 2003;31:333–354. doi: 10.1615/critrevbiomedeng.v31.i4.20. Review. [DOI] [PubMed] [Google Scholar]
- Clutton SM, Townsend KM, Walker C, Ansell JD, Wright EG. Radiation-induced genom-ic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis. 1996;17:1633–1639. doi: 10.1093/carcin/17.8.1633. [DOI] [PubMed] [Google Scholar]
- Cooper B, Schneider S, Bohl J, Jiang Y, Beaudet A, Vande Pol S. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology. 2003;306:87–99. doi: 10.1016/s0042-6822(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Del Seppia C, Choleris E, Luschi P, Thomas AW, Ghione S, Moran GR, Prato FS, Papi F. Exposure to a hypogeomagnetic field, but not to a null magnetic field, reduces stress-induced hypoalgesia in Swiss CD1 mice. Proc Royal Soc Lond B. 2002;269:193–201. doi: 10.1098/rspb.2001.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini L, Abbro L. Bioeffects of moderate intensity static magnetic fields on cell cultures. Micron. 2005;36:195–217. doi: 10.1016/j.micron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Emerit I, Oganesian N, Arutyunian R, Pogossian A, Sarkisian T, Cernjavski L, Levy A, Feingold J. Oxidative stress-related clastogenic factors in plasma from Chernobyl liquidators: protective effects of antioxidant plant phenols, vitamins and oligoelements. Mutat Res. 1997;377:239–246. doi: 10.1016/s0027-5107(97)00080-8. [DOI] [PubMed] [Google Scholar]
- Gandhi OP. Electromagnetic fields: human safety issues. Ann Rev Biomed Eng. 2002;4:211–234. doi: 10.1146/annurev.bioeng.4.020702.153447. [DOI] [PubMed] [Google Scholar]
- Gerashchenko, BI Howell, RW, 2003. Cell proximity is a prerequisite for the proliferative response of bystander cells co-cultured with cells irradiated with gamma-rays. Cytometry, 56A: 71-80, Detecting and measuring chemiluminescense. Available at http://www.lumigen.com/documents/CL_measure.shtml [DOI] [PubMed]
- Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Jasti AC, Wetzel BJ, Aviles H, Vesper DN, Nindl G, Johnson MT. Effect of a wound healing electromagnetic field on inflammatory cytokine gene expression in rats. Biomed Sci Instrum. 2001;37:209–214. [PubMed] [Google Scholar]
- Konopacka M, Rzeszowska-Wolny J. The bystander effect-induced formation of micronu-cleated cells is inhibited by antioxidants, but the parallel induction of apoptosis and loss of viability are not affected. Mutat Res. 2006;593:32–38. doi: 10.1016/j.mrfmmm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Hartmann A, Shephard L, Yang CR, Boothman DA, Bartholomew JA, Morgan WF. Apoptosis, reproductive failure, and oxidative stress in Chinese hamster ovary cells with compromised genomic integrity. Cancer Res. 1998;58:3712–3718. [PubMed] [Google Scholar]
- Little JB, Morgan WF. Guest editors. Special issue of Oncogene. 2003;13 [Google Scholar]
- Liu Z, Mothersill C, McNeill F, Lyng FM, Byun SH, Seymour CB, Prestwich WV. A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiat Res. 2006;166:19–23. doi: 10.1667/RR3580.1. [DOI] [PubMed] [Google Scholar]
- Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ, Boyan BD. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res. 2003;21:326–334. doi: 10.1016/S0736-0266(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Wright EG. Radiation-induced genomic instability and bystander effects: related inflammatory-type responses to radiation-induced stress and injury? A review. Int. J. Radiat. Biol. 2003;79:15–25. Review. [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002a;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry. 2002b;99:169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The Involvement of Calcium and MAP Kinase Signaling Pathways in the Production of Radiation-Induced Bystander Effects. Radiat Res. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- Maguire P, Mothersill C, Seymour C, Lyng FM. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human ker-atinocytes. Radiat Res. 2005;163:384–390. doi: 10.1667/rr3325. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ohnishi T. Contribution of radiation-induced, nitric oxide-mediated bystander effect to radiation-induced adaptive response. Biol. Sci. Space. 2004;18:108–109. [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. Review. [DOI] [PubMed] [Google Scholar]
- Mosse I, Marozik P, Seymour C, Mothersill C. The effect of melanin on the bystander effect in human keratinocytes. Mutat Res. 2006;597:133–137. doi: 10.1016/j.mrfmmm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Lyng F, Lyons M, Cottell D. Growth and differentiation of epidermal cells from the rainbow trout established as explants and maintained in various media. J Fish Biol. 1995;46:1011–1020. [Google Scholar]
- Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, Seymour CB. Involvement of energy metabolism in the production of ‘bystander effects’ by radiation. Br. J. Cancer. 2000;82:1740–1746. doi: 10.1054/bjoc.2000.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Rea D, Wright EG, Lorimore SA, Murphy D, Seymour CB, O'Malley K. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects - implications for cancer. Nature Rev. Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. Review. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Lyng F, Seymour C, Maguire P, Lorimore S, Wright E. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat Res. 2005;163:391–399. doi: 10.1667/rr3320. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Nugent S, Seymour C, Mothersill C. Mitochondrial DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat Res. 2005;585:127–136. doi: 10.1016/j.mrgentox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. Review. [DOI] [PubMed] [Google Scholar]
- Pirisi L, Creek KE, Doniger J, DiPaolo JA. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillo-mavirus type 16 DNA. Carcinogenesis. 1988;9:1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Poon, RC, Agnihotri, N, Seymour, CB, and Mothersill, C, Bystander effects of low doses of ionizing radiation can be modulated by signaling amines, Env Res accepted for publication. [DOI] [PubMed]
- Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo RE, Schiestl RH. Increases in oxidative stress in the progeny of X-irradiated cells. Radiat Res. 2004;162:416–425. doi: 10.1667/rr3238. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res. 2000;153:508–511. doi: 10.1667/0033-7587(2000)153[0508:rcobat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C, Mooney R, Moriarty M, Tipton KF. Monoamine oxidase inhibitors l-deprenyl and clorgyline protect nonmalignant human cells from ionising radiation and chemotherapy toxicity. Br J Cancer. 2003;89:1979–1986. doi: 10.1038/sj.bjc.6601361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. doi: 10.1023/B:CANC.0000031769.14728.bc. Review. [DOI] [PubMed] [Google Scholar]
- Swanson J, Kheifets L. Biophysical mechanisms: a component in the weight of evidence for health effects of power frequency electric and magnetic fields. Radiat Res. 2006;165:470–78. doi: 10.1667/rr3522.1. [DOI] [PubMed] [Google Scholar]
- Werneck MM, Barrientos EM. Fiberoptic transmission of biological signals. Med Prog Technol. 1994;20:59–62. [PubMed] [Google Scholar]
- Yamaguchi S, Ogiue-Ikeda M, Sekino M, Ueno S. Effects of pulsed magnetic stimulation on tumor development and immune functions in mice. Bioelectromagnetics. 2006;27:64–72. doi: 10.1002/bem.20177. [DOI] [PubMed] [Google Scholar]