Abstract
Routine diagnostic X-rays (e.g., chest X-rays, mammograms, computed tomography scans) and routine diagnostic nuclear medicine procedures using sparsely ionizing radiation forms (e.g., beta and gamma radiations) stimulate the removal of precancerous neo-plastically transformed and other genomically unstable cells from the body (medical radiation hormesis). The indicated radiation hormesis arises because radiation doses above an individual-specific stochastic threshold activate a system of cooperative protective processes that include high-fidelity DNA repair/apoptosis (presumed p53 related), an auxiliary apoptosis process (PAM process) that is presumed p53-independent, and stimulated immunity. These forms of induced protection are called adapted protection because they are associated with the radiation adaptive response. Diagnostic X-ray sources, other sources of sparsely ionizing radiation used in nuclear medicine diagnostic procedures, as well as radioisotope-labeled immunoglobulins could be used in conjunction with apopto-sis-sensitizing agents (e.g., the natural phenolic compound resveratrol) in curing existing cancer via low-dose fractionated or low-dose, low-dose-rate therapy (therapeutic radiation hormesis). Evidence is provided to support the existence of both therapeutic (curing existing cancer) and medical (cancer prevention) radiation hormesis. Evidence is also provided demonstrating that exposure to environmental sparsely ionizing radiations, such as gamma rays, protect from cancer occurrence and the occurrence of other diseases via inducing adapted protection (environmental radiation hormesis).
Keywords: radiation hormesis, adaptive response, LNT
INTRODUCTION
Ionizing radiation spans the universe in which we reside (Bonner 2003). There are two basic forms of ionizing radiation: electromagnetic and particulate. Electromagnetic radiation is comprised of uncharged photons (entities without mass) that interact with electrons in matter causing ionizations if photon energy is high enough. Examples of ionizing electromagnetic radiations are X-rays and gamma rays. Examples of particulate ionizing radiation are alpha and beta particles emitted by radioisotopes and protons ejected from the sun. Neutrons do not directly cause ionizations but cause them indirectly through secondary charged particles such as protons (e.g., from water in biological tissue) that are dislodged by neutrons.
Natural background ionizing radiation on earth comes from the following three sources: the sun (solar radiation), outer space (cosmic rays), and terrestrial sources (e.g., radionuclides in our bodies and environment, and radon in the home) (NCRP 1997). While most solar radiation is electromagnetic, the sun also produces particulate radiation (solar cosmic rays), including protons, which vary with the solar cycle.
All organisms on earth are constantly bombarded by cosmic radiation from outside our solar system. This radiation is comprised of charged particles ranging in atomic mass from protons to iron nuclei. These particles interact in the atmosphere creating secondary radiation that rains down and includes X-rays, electrons, protons, alpha particles, neutrons, pions, and muons. Our exposure to cosmic rays increases each time we take an airline flight. Persons living at high elevations such as in Denver, Colorado, and Salt Lake City, Utah, receive higher exposures to cosmic rays than do persons residing in Miami, Florida, or New Orleans, Louisiana.
Natural radioactivity (the capacity to emit particulate or electromagnetic ionizing radiation forms) is everywhere on earth. All organisms on earth are continuously exposed to varying amounts of natural radiation. We humans are irradiated from: radioactivity in our bodies (e.g., associated with potassium-40), natural radioactivity in ingested foods (e.g., associated with carbon-14), exposure to radiation emanating from soils and rocks (e.g., from uranium and thorium isotopes), and exposure in our homes and businesses to radon and its radioactive daughter radionu-clides. Thus, we humans are continuously exposed to radiation arising from naturally occurring terrestrial radioactivity and from the cosmos. This is true prior to birth and through one's entire life.
Diagnostic X-rays (chest X-rays and computed tomography scans) and other sources of radiation used in nuclear medicine diagnostic procedures are also sources for our exposure to low-level ionizing radiation. Both exposure to natural background sparsely ionizing radiation and exposure to diagnostic sparsely ionizing radiation sources are likely playing a beneficial role in the maintenance and preservation of life on earth through suppressing genomic-instability-associated diseases such as cancer. This topic is partly the focus of this paper. An additional focus is on the use of low doses and dose rates of sparsely ionizing radiation in curing existing cancer.
The potential for severe radiation damage is generally evaluated based on what is called linear energy transfer (LET), which is just the average energy loss when penetrating a small thickness of material (e.g., tissue). Low-LET radiations include X-rays, gamma rays, and beta particles. These radiation forms deposit relatively small amounts of energy when penetrating a small thickness of tissue. High-LET radiation (e.g., alpha particles and neutrons) deposit more energy in the indicated small thickness of tissue. High-LET radiations usually cause more biological damage locally in tissue than low-LET radiation.
It is important to be aware that small amounts of radiation kill only a few cells and those cells are generally replaced without harm in humans and other mammals. Radiation also produces sublethal damage to our cells, and most such damage is repaired without any significant error (e.g., error free). However, some cells commit repair errors (i.e., misre-pair leading to mutations). Mutations represent a form of genomic instability. A certain amount of instability is tolerated by cells and the instability can propagate over subsequent cellular generations. Cells with threatening instability may commit suicide (apoptosis) or may be eliminated via the immune system. Uncontrolled instability in the genome can result in cancer and other diseases.
The oxygen we breathe is by far the greatest natural cause of cellular damage–many orders of magnitude greater than other natural causes (Pollycove and Feinendegen 2003). All living mammals have a system of protective processes that prevents, repairs, and removes cell damage. Radiation primarily affects the components of this protective system. Low doses activate protection resulting in fewer mutations, neoplastic transformation, and cancers, while high doses suppress some of the protection resulting in more of the indicated stochastic effects (Feinendegen et al. 2004; Scott 2005a,b, 2006a,b).
The recently released BEIR VII Report (Phase 2) has implicated diagnostic X-rays (e.g., chest X-rays, mammograms, CT scans) and nuclear medicine diagnostic procedures as causing harm through inducing excess cancers (NRC 2006). This view is based on the linear-no-threshold (LNT) hypothesis of radiation carcinogenesis, which states that cancer risk increases as a LNT function of radiation dose, no matter how small. Relative risk (RR) therefore is expected to increase linearly without a threshold from a value of 1 at natural background radiation exposure (usually assigned a dose of zero). RR is just the risk after exposure to radiation divided by the risk when exposed only to natural background radiation. Thus, without any radiation exposure beyond the natural background level, RR would equal 1 (normal risk). With excess cancers induced by the above natural background irradiation, RR would be greater than 1 under the LNT assumption. However, according to the LNT hypothesis, even natural background radiation is harming us. Reducing background radiation exposure would be expected to reduce risk, although this appears not to be the case as is discussed later.
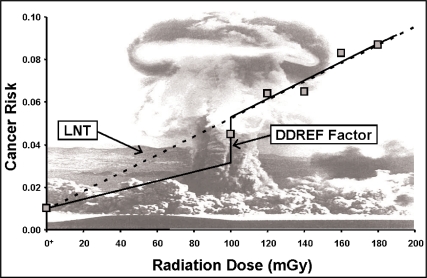
Cancer risk estimates based on the LNT hypothesis (Figure 1) are mainly based on extrapolating high-dose cancer mortality data acquired following the nuclear blasts that took place in Hiroshima and Nagasaki, Japan, to low doses (NRC 2006). A LNT cancer risk curve is fitted to the high-dose cancer frequency data, as was done in the BEIR VII Report (NRC 2006). For evaluating cancer risk after low doses and dose rates, a low-dose and dose-rate effectiveness factor (DDREF) is then used to reduce the slope of the curve by a fixed amount (Figure 1). By using the DDREF approach, the BEIR VII Report essentially dismissed the radiation horme-sis phenomena since only positive slopes are permitted for the dose-response curve. With hormesis, low doses protect against cancer and other diseases leading to a negative initial slope for the dose-response curve. However, high doses fail to induce protection and even inhibit protection causing risk to then increase as dose increases further, leading to what has been called a J-shaped (or U-shaped) hormetic dose-response curve.
FIGURE 1.
LNT risk function which is usually based on data derived from high doses delivered at high rates from the atomic bombings in Hiroshima and Nagasaki, Japan. The high-dose LNT curve is reduced by a DDREF when evaluating the risks at low doses and dose rates. Even so, the slope of the dose-response curve can never be negative (i.e., a hormetic response curve). The notation O+ is used to represent the natural background radiation dose, presented as though it were located at zero.
In contrast to the BEIR VII Report use of the LNT hypothesis when assessing low-LET radiation-associated cancer risk at low doses and dose rates, the recent French Academies report related to LNT dismissed the LNT hypothesis for low-LET radiation doses less than 100 mGy and found radiation hormesis to be plausible (Tubiana 2005; Tubiana et al. 2005).
This paper presents evidence that we are unlikely to be harmed by infrequent applications of diagnostic X-rays (from a chest X-ray machine, mammogram, or CT scan), by most routine nuclear medicine procedures or by elevated natural background radiation (including radon in our homes). More importantly, this paper provides evidence that low levels of low-LET radiation (e.g., X-rays or gamma rays) received from natural and medical sources protect us from cancer and other diseases via stimulating a system of known protective processes. Similar protection also appears to be associated with combined exposure to low doses and dose rates of alpha plus gamma radiation (as occurs for radon in the home).
LOW-DOSE/DOSE-RATE LOW-LET RADIATION-INDUCED SYSTEM OF PROTECTION
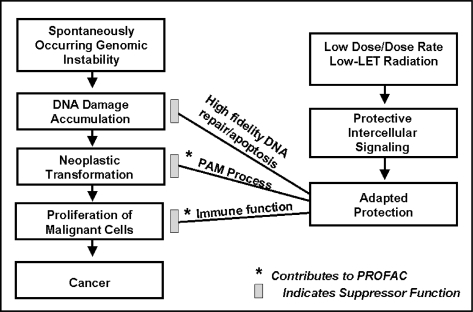
As previously indicated, low doses and dose rates of low-LET radiation activate a system of cooperative protective processes in the body. The protective processes include (1) defenses such as scavenging of reactive oxygen species and other toxins, (2) presumably p53 related activated high-fidelity DNA repair/apoptosis, (3) a novel auxiliary protective apoptosis mediated (PAM) process that selectively eliminates aberrant cells, and (4) induced immunity (Liu et al. 1994; Liu, 2004). The PAM process has been demonstrated to involve reactive oxygen and nitrogen chemical species, specific cytokines (e.g., transforming growth factor beta in the case of fibroblast cells), and can occur independently of the p53 gene (Scott 2004, 2005a; Scott et al. 2006). The indicated protective processes, which are activated by low doses and dose rates of low-LET radiation or low plus high-LET radiation appear to be inhibited by moderate and high doses (a characteristic of hormetic effects). The PAM process appears not to be activated by high-LET alpha radiation alone (Scott 2004, 2005a, 2006a). However, more research is needed to confirm this.
In this article the idea is put forth that low doses and dose rates of diagnostic X-rays, gamma rays, and beta radiation can prevent cancer occurrence via stimulating selective removal of precancerous neoplasti-cally transformed cells that could otherwise lead to cancer. In the next section, we briefly discuss publications which indicate that low doses of low-LET radiation are protecting us from mutations, neoplastic transformation, and cancer (including cancer metastasis) and other diseases.
EVIDENCE THAT LOW-DOSE RADIATION PROTECTS US
Low doses of low-LET radiation (gamma or X-rays) have been demonstrated to
Induce defense such as detoxification of reactive oxygen species (for review, see Feinendegen et al. 2004).
Induce high-fidelity repair of DNA damage (Joiner et al. 1999; Rothkamm and Löbrich 2003).
Protect from chromosomal damage from a subsequent high radiation dose (Wolff et al. 1988).
Protect from spontaneous mutations occurrence in vivo (Hooker et al., 2004; Scott et al. 2006).
Protect from spontaneous neoplastic transformation occurrence in vitro (Azzam et al. 1996; Redpath et al. 2001; Redpath et al. 2003; Redpath 2005; Ko et al. 2004; Elmore et al. 2005).
Protect from spontaneous cancers in animals (Sakai 2003).
Extend tumor latency in cancer-prone mice (Mitchel et al. 2003; Mitchel 2004, 2005)
Activate the immune response (Liu et al. 1987; Makinodan and James 1990; Sakamoto et al. 1997; Liu 2003, 2004) and suppress lung and lymph node metastasis in vivo (Hosoi and Sakamoto 1993; Hashimoto et al. 1999; Sakamoto 2004).
Suppress spontaneous cancers in humans (Howe, 1995; Rossi and Zaider 1997; Scott 2005a, 2006a).
Protect from some diseases other than cancer (Luckey 1991; Wang et al. 2005).
The low-LET radiation doses that protect us fall into a presently not-well-defined dose zone which is dose-rate and exposure-duration dependent (Scott 2004, 2005a; Scott et al. 2006). For brief exposure at a high rate to X-rays (28-kVp, 60-kVp, or 250-kVp) and for neoplastic transformation the protective zone includes doses in the 0.5 mGy to 10 mGy range (Scott 2004, 2005a). The 28-kVp X-rays are representative of mammographic-energy X-rays (Ko et al. 2004). For high-energy, gamma-ray photons, the protective zone includes doses in the range 1 mGy to 100 mGy. For protracted exposure of humans, the zone is increased to include total doses over several hundred miligray as discussed later, related to multiple applications of fluoroscopy and mammography.
Doses currently associated with applications of X-rays and other routine diagnostic radiations fall in the protective zone (Tables 1 and 2) and therefore are likely protecting us from cancer and some other diseases. Unfortunately, because of the BEIR VII Report (NRC 2006) claim that any amount of radiation is harmful, many citizens are now terrified of having to undergo diagnostic chest X-rays, mammograms, CT scans, or nuclear medicine diagnostics. Using a LNT risk function, the BEIR VII Report (NRC 2006) concluded that such diagnostic treatments harm us through inducing cancers. To the contrary, research results presented in this paper and elsewhere (Scott 2005a, 2006a) suggest that some precan-cerous neoplastically transformed cells in the body disappear (medical radiation hormesis) as a result of the low-level, low-LET radiation exposure associated with diagnostic X-rays. Multiple X-rays (e.g., from CT scans, mammograms, chest X-rays) at appropriate intervals (not yet determined) would be expected to increase the efficiency of removal of the neoplastically transformed cells as well as other genomically unstable cells. Repeated low doses of X-rays likely over and over stimulate the transient PAM process and immunity. The indicated low-dose-radiation-induced system of protection is illustrated in Figure 2. The protection factor (PROFAC) in Figure 2 is discussed later. Once activated, the indicated system of protection could eliminate existing precancerous cells in the body, e.g., those that arise from cigarette smoking. Activating the protective system by low-dose, low-LET radiation could also protect us from harm from other genotoxicants we are exposed to in the environment and the workplace.
TABLE 1.
Doses from routine diagnostic X rays and possibility of hormesis induction
| Number of X rays | Dose rangea | Hormesis likely? |
|---|---|---|
| <5 | 0.01 mGy – 30 mGy | > 0.01 mGy Yes* |
| 5-14 | 0.1 mGy-50 mGy | Yes* |
| ≥ 14 | 1 mGy – 230 mGy | Yes* |
Scott (2005a); Scott et al. (2006)
TABLE 2.
Doses from typical diagnostic radiation sources in the United States and possibility for hormesis inductiona
| Source | mGy | Hormesis likely? |
|---|---|---|
| Dental, full-mouth (X ray) | 0.17 | Yesb |
| Chest X ray | 0.25 | Yesb |
| Mammograms (X ray) | 4 | Yesb |
| CT scan, head (X ray) | 20 | Yesb |
| CT scan, body (X ray) | 60 | Yesb |
| Thyroid scans: | ||
| Iodine-131 (β + γ radiation) | 50-100 | Yesb |
| Iodine-123 (γ radiation) | 30-50 | Yesb |
| Technetium-99 (β radiation) | 10 | Yesb |
FIGURE 2.
Low-dose, low-LET-radiation-induced system of protection against spontaneous cancers. The indicated protective components are features of the HRR model. Increasing DNA fidelity influences the slope parameter KL while the PAM process and induced immunity influence the PROFAC.
HORMESIS-BASED VS. LNT-BASED RELATIVE RISK
As already indicated, cancer RR after low doses of ionizing radiation of any type is most often assessed based on the LNT hypothesis. However, in the case of hormesis (a beneficial effect of irradiation, with cancer cases decreasing below the spontaneous level), RR would be less than 1 after low radiation doses in excess of natural background radiation, when evaluated relative to the cancer risk at background radiation exposure. The hormetic response is presumed to be associated with the system of radiation-induced protective processes (PAM process [presumed p53-independent], immune system stimulation, and activated high-fidelity DNA repair/apoptosis [presumed p53-dependent]), leading to a reduction in cancer incidence below the spontaneous incidence (Scott 2004, 2005a, 2006a,b).
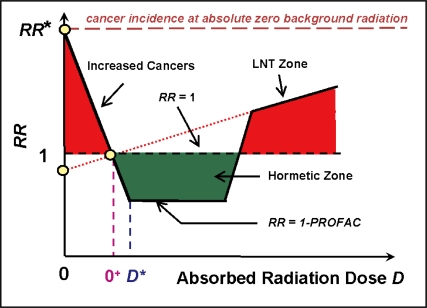
Figure 3 shows the expected RR dose-response curve general shape based on our new quantitative hormetic RR (HRR) model (Scott 2006a,b) when radiation doses range from absolute zero, 0, to above the current natural background exposure level, O+. Figure 3 is used to explain environmental exposures to low- or high-radiation doses but also can be used for other forms of exposure (e.g., occupational exposure of nuclear workers). With the HRR model, low doses and dose rates of radiation (low-LET component only) are considered to stimulate the above indicated system of protective processes causing RR to decrease progressively to well below the spontaneous level of 1 (at natural background exposure) for radiation doses somewhat above the natural background radiation dose of O+ (Figure 3). The total radiation dose D is made up of a low-LET component DL and high-LET component DH. When the total radiation dose D decreases below the natural background radiation level of O+, RR is expected to increase linearly as the low-LET dose component DL decreases due to a progressive loss of adapted protection. The loss occurs as DL falls below the individual-specific threshold (stochastic) for activating the protective processes that contributed to adapted protection (Scott 2006b). Currently, a uniform distribution of these thresholds has been assumed over the closed dose interval [0,D*], where D* is the minimum total radiation dose for which the system of protection is activated by the low-LET component DL* in each irradiated person.
FIGURE 3.
Basic features of the HRR model. Doses 0 and O+ represent absolute zero radiation and natural background radiation dose respectively. The dose D* is the dose rate and radiation quality dependent dose at which the hormetic effect is maximal. Individual specific thresholds for activating the system of protective processes associated with radiation hormesis are currently assumed to be uniformly distributed over the closed interval [0,D*]. RR is projected to increase linearly from 1 - PRO-FAC at the dose of D* (> 0+) to a value of 1 as the dose D is reduced to background radiation dose O+. For further decreases in dose D below O+, RR is projected to increase to a value RR* (at absolute zero radiation) when evaluated relative to the risk at O+.
The dose zone [0,D*] is called Transition Zone A since it contains the stochastic thresholds for activating the system of protection that contributes to the radiation adaptive response (Scott 2005a, 2006b; Scott et al. 2006). Above the total dose D*, RR is roughly constant at RR = 1 – PRO-FAC, over a Zone of Maximal Protection that is relatively wide after low-rate exposure and narrow after high-rate exposure. Then at higher doses, protection is lost (PAM process and immune system stimulation) causing a steep rise in the dose-response curve (Transition Zone B). Just above a dose where protection is lost in each irradiated person, the curve then enters the LNT Zone that has been investigated in many epidemiological studies (NRC 2006) and inappropriately used to justify a LNT extrapolation of cancer risk down to the dose O+ (the natural background exposure level). For the LNT Zone, immunity and the PAM process are considered to be maximally suppressed. Doses in this zone and higher (e.g., doses associated with conventional fractionated therapy individual dose fractions) may therefore promote metastasis of existing cancer.
For Transition Zone A, changes in RR are determined by DL (Scott 2006b). For the Zone of Maximal Protection, RR is essentially independent of dose. For Transition Zone B, RR depends both on DL and DH. For the LNT Zone RR also depends on DL and DH (Scott 2006a,b).
The related cancer RR equation for the HRR model depends on the radiation exposure scenario. The solution provided below applies for combined exposures to low- and high-LET radiation for D ≥ O+. At and above natural background radiation exposures the cancer RR is characterized by the following equation:
for background radiation exposure (D = O+), and
| (1) |
for doses > background.
Here f(B) represents the quotient (1 − B)/B, where B is the baseline cancer frequency (incidence of mortality depending on the endpoint modeled). KL and KH are called slope parameters and are associated with the low- and high-LET components of the radiation, respectively. For example, with combined exposure to alpha and gamma radiations (as occurs for radon in the home), KL would be associated with the gamma rays and KH with alpha radiation. Generally KH > KL (Scott 2006a) in Equation 1 which was derived from a corresponding equation for neo-plastic transformation (Scott 2004, 2005a, 2006a) by replacing the spontaneous transformation frequency T0 with the baseline cancer frequency (incidence or mortality) B and using different slope parameters for cancer (uppercase “K”) than were used for transformation (lowercase “k”). Justification for this approach is based on the observation that the RR dose-response curve for neoplastic transformation and for cancer induction appear to have the same shape (Redpath et al. 2001; Scott 2005b, 2006a).
The PROFAC (protection factor) in Equation 1 accounts for radiation hormesis associated with immune system stimulation and activation of the PAM process. However, it relates only to the low-LET component of the dose. When only high-LET alpha radiation is involved, PROFAC is presumed to be zero (Scott 2006a). For cancer mortality considerations, the PROFAC represents the expected proportion of deaths avoided as a result of radiation hormesis among those lives that would otherwise have been lost to cancer. For cancer incidence considerations, the PROFAC represents the expected proportion of cancer cases avoided as a result of radiation hormesis among those that would otherwise have occurred. The PRO-FAC differs for different cancer types and can differ for different exposure scenarios. For results that are presented later, it has been assumed that similar PROFACs apply to cancer incidence and cancer mortality. Thus, PROFACs based both on cancer incidence and cancer mortality have been used in evaluating expected lives saved due to radiation hormesis.
Regarding Equation 1, for low doses and dose rates (near natural background levels), the term (1 – PROFAC) is expected to predominate for exposure only to low-LET radiation as well as for combined exposure to low- and high-LET radiation. In this case,
| (2) |
For exposure only to low-LET X-rays or gamma rays, RR = 1 at the natural background radiation dose and then drops to a value 1 – PROFAC for doses that active the previously indicated protective processes. Here it is assumed that the smallest doses of interest above the natural background radiation dose are sufficient to stimulate the protective processes. Otherwise, a more complicated approach is needed related to evaluating stochastic threshold distributions for activating the system of protective processes (Scott et al. 2004; Scott 2006a). Currently available information suggests that the protective PAM process may be stimulated by low-LET radiation doses as low as 0.02 mGy which can be obtained from monthly background radiation doses in some regions of the globe (Scott, 2005a). This conclusion is based on a study of radiation-induced inversion mutations in mice (Hooker et al. 2004). For neoplastic transformation, doses as low as 0.4 mGy have been demonstrated to be protective (Scott 2005a; Scott et al. 2006). However, there are no data for lower doses except for exposure of controls to background radiation.
Moderate and high doses can inactivate the PAM process and suppress (rather than stimulate) the immune system (Scott 2006a,b; Hashimoto et al., 1999) leading to increased radiation-associated cancers. Thus, the increased incidence of cancer at moderate and high doses relates to the loss of protection against stochastic effects. The RR at moderate and high doses therefore increase as dose increases. The range of doses over which RR < 1 is expected to increase when the radiation is given at a low rate and over an extended period (Scott 2004, 2005a, 2006a,b). This is also expected to be the case for exposure to multiple, small doses, each of which fall in the dose zone where the protective processes are activated (i.e., hormetic zone). This protective dose zone depends on the type of radiation and for photons depends on photon energy (Scott 2005a, 2006a; Scott et al. 2006). For a single dose of diagnostic X-rays delivered at a high rate, this protective zone associated with the PAM process includes doses in the range from 1 mGy to 10 mGy (Scott, 2005a; Scott et al. 2006). For brief exposures at a high rate to high-energy gamma rays, doses as high as 100 mGy fall in the hormetic zone (Scott 2005a; Scott et al. 2006).
Both RR and standardized mortality ratio (SMR) are used in this paper to estimate the PROFAC for in vivo considerations where both the PAM process and immune system stimulation are presumed to contribute to protection against cancer. The SMR in some cases is used as an estimate of RR, although these statistics can differ depending on dose lagging and other assumptions. Odds ratio can also be used as an estimate of RR for rare diseases.
For exposures at below natural background radiation levels, the RR is predicted to increase linearly from 1 at current natural background radiation exposure to a value RR* at absolute zero natural background. As dose is increased from the background level to the dose D* in Figure 3, RR is predicted to decrease from 1 at natural background to 1 – PROFAC. However, the PROFAC can vary for different individuals, so as used in this paper PROFAC represents a populations-specific average.
The HRR model is pragmatically applicable to all types of exposures, be they acute or protracted or fractionated, and is based on high- and low-LET absorbed radiation doses, rather than on a weighted combination of these doses.
IMPLICATIONS OF THE DOSE-INDEPENDENT ZONE
With the HRR model, there is a relatively large dose region (Figure 3) over which RR is suppressed below 1 and relatively independent of dose (flat portion of curve). This has important implications for ecological studies of cancer occurrence in elevated radiation environments. Ecological studies of radiation-induced cancer have been criticized by advocates of the LNT hypothesis because radiation doses associated with such studies have large errors, thereby preventing the researchers from comfortably calculating excess RR per unit dose (a widely used, risk-assessment tool based on the LNT hypothesis). However, if there is a large dose region over which RR is independent of dose, then dose errors are far less important and LNT does not apply!
With the HRR model and for low doses delivered at low rates over prolonged periods, the dose-independent region of suppressed RR is expected to include doses to several hundreds of milligray and possibly much higher. For such a dose-independent region one needs only to estimate the PROFAC in order to fully characterize the radiation response. In doing so, the expected number of cancer deaths (or cases) avoided (due to radiation hormesis) per each 100 deaths (or cases) that would otherwise occur is simply given by 100 × PROFAC, when PROFAC is evaluated for the type of cancer death (or case) of interest (e.g., lung cancer, leukemia, etc.). Assuming a binomial distribution for the number of lives saved (cancer deaths avoided) due to radiation hormesis, the standard deviation for the expected lives saved is given by the square root of the variance, where the variance equals 100 × PROFAC(1 – PROFAC).
DATA SUPPORTING THE HRR MODEL
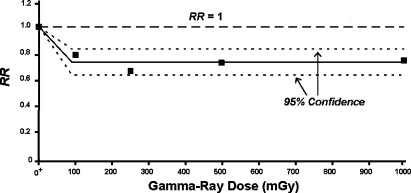
A similar curve shape as in Figure 3 (HRR model) for doses > D* where RR is suppressed below 1 at a level of 1 – PROFAC for a wide range of doses has been observed for lung cancer induction by gamma-ray exposure of a very large population (> 15,000) of laboratory mice, based on data from Ullrich et al. (1976). For the indicated data, RR is adequately described by a curve that decreases from 1 at the background dose O+ to a value of 1 – PROFAC at near 100 mGy and remains essentially constant for doses to 1,000 mGy. The indicated data are presented in Figure 4. The indicated suppression of lung cancer by induced protective processes is highly significant (p < 6 × 10−8; based on average RR = 0.735 ± 0.05 for the four nonzero dose groups). The average value for the PROFAC based on these data is 0.265 ± 0.05. Thus on average, about 27% of the spontaneous lung cancers were prevented by radiation hormesis for doses over the range 100 to 1,000 mGy. The central solid line in Figure 4 is just the average of the four RR values plotted at doses > 0 and corresponds to 1 – PROFAC. The dashed curves are approximate 95% confidence intervals assuming a dose-independent normal distribution for RR for doses in the range 100 to 1,000 mGy. The data are consistent with the existence of a large dose-independent zone (correlation coefficient R2 = 0.03 for RR vs. dose for doses ≥ 100 mGy). There is no evidence for doses < 1,000 mGy being associated with an increase in cancer risk as would be predicted using the LNT hypothesis! In a later paper the researchers (Ullrich and Storer 1979) indicated having detected a systematic error related to lung tumor detection. However, correcting such a systematic error would not be expected to alter the RR curve shape presented here.
FIGURE 4.
Lung cancer RR based on more than 15,000 mice exposed at Oak Ridge National Laboratory to gamma rays based on data reported by Ullrich et al. (1976). The notation O+ is used to indicate the natural background radiation dose, presented as though it were located at zero.
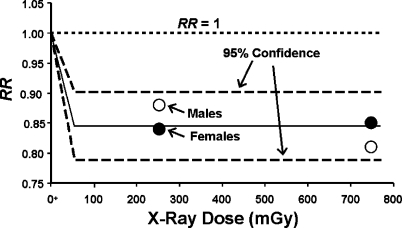
The dose-response curve shape in Figure 4 has also been demonstrated for lung cancer induction in humans exposed to fractionated low-LET radiation for absorbed doses up to about 1,000 mGy of diagnostic X-rays as shown in Figure 5 (Canadian Fluoroscopy Cohort Study, Howe 1995). Data points are presented separately for males and females. Published dose bins (Howe 1995) were used with the data points plotted at the midrange of the bins. The low-LET data used were based on multiple applications of diagnostic X-rays given to TB patients. The data in Figure 5 are consistent with the notion that fractionated exposures to diagnostic X-rays over and over stimulate the removal of precancerous neoplastically transformed cells from the lung and thereby reduce the risk of lung cancer (medical radiation hormesis). There is no evidence that fractionated diagnostic X-ray doses ≤ 1,000 mGy are causing excess lung cancers as would be predicted based on the LNT hypothesis! Instead, there is strong evidence for medical radiation hormesis.
FIGURE 5.
Applications of the HRR model to lung cancer data for humans (TB patients) exposed to fractionated X-ray (diagnostic) doses, based on data reported by Howe (1995). Data for males and females were jointly analyzed. The two dashed curves indicate the 95% confidence region RR = 1 -PROFAC. The central curve is based on the average value for RR for the four data points. Such averaging is justified based on the HRR model. The notation O+ is used to indicate the natural background radiation dose, presented as though it were located at zero.
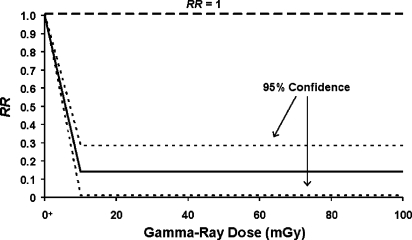
A similar curve shape has also been used (Scott 2004) to characterize lung cancer risk in Mayak plutonium facility workers chronically exposed at low rates over years to gamma radiations based on data reported by Khokhryakov et al. (1996). The results presented here were corrected for exposure to alpha radiation (Scott 2006a) using the HRR model. The dose-response curve and 95% confidence region is presented in Figure 6 and shows the high degree of protection that appears to be associated with exposure over years at low rates to gamma rays. The average value for PROFAC was 0.86 ± 0.07 (Scott, 2004), similar to the very large but controversial PROFAC (> 0.95) reported for chronic gamma-irradiation-induced protection against cancer in Taiwanese citizens (Chen et al. 2004) residing in apartments built of steel contaminated with cobalt-60 (a gamma-ray source). The gamma-ray dose appears not only to have protected against cigarette-smoking-associated lung cancers in the Mayak workers, but also against alpha-radiation-induced lung cancer (Scott 2006a). Russian national statistics were used to obtain risk estimates for an unexposed population. The results in Figure 6 are consistent with the view that chronic exposure of humans at a very low rate over years to gamma radiation over and over activate the system of transient protective processes that contribute to radiation adaptive response (hormesis).
FIGURE 6.
Applications of the HRR model to lung cancer mortality data for Mayak workers chronically exposedover years to gamma and alpha radiation at low rates. Results presented were adjusted for the influence of alpha irradiation (Scott 2006a). Only the gamma-ray dose (for an arbitrary dose range) is therefore indicated. The notation O+ is used to indicate the natural background radiation dose, presented as though it were located at zero.
Small X-ray doses have been demonstrated to suppress lung metastasis of squamous carcinoma cells transplanted into mice (Sakamoto 2004). The dose-response curve shapes for suppressing lung metastasis in vivo and for suppressing neoplastic transformation in vitro by low doses of X-rays (or gamma rays) are quite similar. The indicated curves have shapes similar to that for RR in Figure 3 for doses ≥ 0+. Low doses caused a reduction both in transformation frequency and lung metastasis below the value for controls not receiving any radiation exposure. The PAM process and induced high-fidelity DNA repair are thought to be responsible for the in vitro suppression of neoplastic transformation (Scott 2004). However for transplanted squamous carcinoma cells, induced DNA repair could not explain suppression of lung metastasis. More likely contributors to the in vivo suppression of metastasis are activation of the PAM process and enhanced immunity. Moderate and high doses, however, inhibit the PAM process and suppress immunity so that the dose-response curve for lung metastasis would be expected to rise to above the value for controls, as was observed by Sakamoto (2004).
ADDITIONAL EVIDENCE THAT RADIATION EXPOSURE OF HUMANS IS PREVENTING CANCER
Values of PROFAC significantly > 0 for cancer occurrence (or cancer mortality) demonstrate a suppression of cancer (i.e., cancer prevention). Estimates of PROFAC for a number of irradiated populations (populations exposed to elevated background radiation and nuclear workers) have been derived based on cancer mortality data reported by Jaworowski (2001) and are presented in Table 3 along with estimates based on additional data. Radiation exposures were presumed to have occurred in the hormetic zone in cases where RR or the SMR was < 1. All indicated PROFAC values were significantly > 0 (p < 0.05). Results for seven populations all exposed to low- or low- plus high-LET radiation at low rates or to fractionated diagnostic X-rays are presented in Table 3. PROFAC values range from 0.15 (15% of spontaneous cancers prevented) for Chernobyl accident recovery workers to 0.86 (86% of spontaneous cancers prevented) for Mayak workers. Even residing in U.S. states with high natural background appears to suppress cancer occurrence (PROFAC = 0.15).
TABLE 3.
Central estimates of the radiation-hormesis-related protection factor (PROFAQ against cancer in humans
| Group | Effect | Radiation types | PROFAC |
|---|---|---|---|
| Chernobyl accident recovery workers (Ivanov et al, 2001) | Cancers | Low- plus high-LET | 0.13a |
| USA, residents of high background states (Frigerio and Stowe, 1976) | Cancers | Low- plus high-LET | 0.15a |
| British medical radiologistsb after 1955–1979 (Berrington et al, 2001) | Cancers | Low-LET | 0.29a |
| High residential radon, USA (Cohen, 1995) | Cancers | Low- plus high-LET | 0.35a |
| Canadian nuclear industry workers (Gribbin et al, 1992) | Leukemia | Low- plus high-LET | 0.68a |
| USA DOE facilities workers (Gilbert et al, 1993) | Leukemia | Low- plus high-LET | 0.76a |
| Russian Mayak plutonium facility workers (Scott, 2006a) | Lung cancer | Low- plus high-LET | 0.86a-c |
PROFACsignificantly > 0 (p < 0.05).
Evaluated relative to all men in England and Wales.
Bayesian posterior mean with an associated standard deviation of 0.07.
As previously indicated, the product 100 × PROFAC gives the expected number of deaths from cancer avoided due to radiation-induced adaptive protection (hormesis) for each 100 cases that would have otherwise occurred. Thus, for Mayak workers, 86 lung cancer deaths are expected to have been prevented for each 100 lung cancer deaths that would have otherwise occurred in the absence of their chronic exposure to gamma radiation. This is a pronounced level of protection against normally occurring harm, including harm associated with cigarette smoking.
EXPECTED IMPACT OF AGE AT EXPOSURE ON THE PROFAC
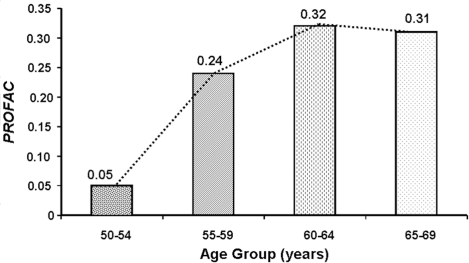
DNA repair fidelity is known to be reduced with increasing age (Szczesny et al. 2003). Thus, genomic instability is expected to increase as we age because of reduced DNA fidelity. However, increasing genomic instability would be expected to be associated with an increased role of the PAM process and immune system stimulation in protecting against genomic-instability-associated diseases such as cancer. The PAM process involves signaling between normal and aberrant cells. The higher the concentration of genomically unstable cells the stronger the signaling associated with the PAM process is expected to be, once signaling is initiated (Scott 2004). Thus, one would expect the PROFAC to increase as age increases for a given genomic-instability-associated disease. This appears to be the case based on PROFAC estimates presented in Figure 7 for radiation-induced protection against breast cancer after multiple mammo-grams (related to diagnostics for breast cancer occurrence). The data are based on Nyström et al. (2002). The results appear to indicate that the aged benefit more from induction of the PAM process and immunity than young adults. Whether or not the very young will benefit from the PAM process is unclear. Induced immunity would be expected to prove beneficial even for the very young.
FIGURE 7.
Proportion of breast cancer prevented (PROFAC) due to radiation hormesis as a function of age at exposure to diagnostic X-rays. Based on data for fractionated exposure (mammograms) of humans during breast cancer screening (Nyström et al. 2002).
THERAPEUTIC RADIATION HORMESIS IMPLICATIONS
The relatively large PROFAC values in Figure 7 are consistent with the view that the transient protective processes (PAM process and induced immunity) can be activated over and over via fractionated exposures to low doses in the hormetic zone. Such repeated doses would also be expected to remove a fraction of the existing cancer cells (therapeutic radiation hormesis at low doses). However, cancer cells are known to resist undergoing apoptosis (PAM process resistance) (Scott, 2004). Ongoing cancer therapy and cancer prevention research is leading to new discoveries of agents that sensitize cancer cells to undergo apoptosis. One such agent is the natural phenolic compound resveratrol (3,4′,5,-tri-hydroxystilbene) which is present in significant amounts in red wine, grapes, peanuts, green vegetables, in other edible spermatophytes, and in many oriental herbal beverages (e.g., green tea) and medicines (Fiore et al., 2005; Sgambato et al. 2000).
Resveratrol has been shown to potentiate the apoptotic effects of gamma radiation, cytokines (e.g., TRAIL), and chemotherapeutic agents (Aggarwal et al. 2004). In addition to beneficial cardiovascular effects, resveratrol exhibits anticancer properties, as suggested by its ability to suppress proliferation of a wide variety of tumor cells, including myeloid and lymphoid cancers; multiple myeloma; cancers of the stomach, prostate, breast, colon, thyroid and pancreas; melanoma; squamous cell cancinoma in the head and neck; ovarian carcinoma; and cervical carcinoma (Aggarwal et al. 2004).
The cancer suppressive effects of resveratrol involve signaling through multiple pathways (e.g., to apoptosis) and are mediated via the following (Aggarwal et al. 2004): (1) cell cycle arrest; (2) upregulation of p21Cip1/WAF1, p53, and Bax; (3) downregulation of survivin, cyclin D1, cyclin E, Bcl-2, Bcl-xL and cIAPs; and (4) activation of caspases. Resveratrol has been demonstrated to suppress the activation of transcription factors that include AP-1, NF-κB, and Egr-1; to inhibit protein kinases including IkBα kinase, JNK, MAPK, Akt, PKD, PKC, and casein kinase II; and to down-regulate products of genes that include IL-1, IL-6, IL-8, AR, COX-2, 5-LOX, VEGF, and PSA.
The ability of resveratrol to trigger apoptosis (likely the PAM process) has been established in different human tumor cell lines (Hsieh and Wu 1999; Clement et al. 1998; Surh et al. 1999; Ahmad et al. 2001; Dorrie et al. 2001; Tinhofer et al. 2001). Joint applications of fractionated or protracted low-dose irradiation (low-LET) in combination with applications of resveratrol may lead to enhanced selective killing of cancer cells (genom-ically unstable cells selectively removed via the PAM process). Such low-dose combined therapy would likely be preferred by cancer patients over current high-dose radiation and chemotherapy which are associated with severe side effects. However, new research is needed to develop optimal dosing schemes. Common low-LET radiation sources used in medical diagnostics could be used in this form of combined therapy, including those used in nuclear medicine. Further, low-dose/dose-rate radioim-munotherapy could be employed in combination with applications of apoptosis-sensitizing agents (for selectively sensitizing cancer cells), such as resveratrol, in curing cancer while minimizing side effects. Other plant polyphenols that also sensitize cancer cells to undergoing apoptosis are genistein, curcumin, emodin, and flavopiridol (Garg et al. 2005). Multiple pathways to apoptosis may be associated with the indicated sensitizers since multiple pathways are known to be associated with resveratrol-induced apoptosis (Aggarwal et al. 2004). These pathways include the Fas pathway, mitochondrial pathway, Rb-E2F/DP pathway, p53-activation pathway, the ceramide-activation pathway, the tubulin-polymerization pathway, and the Adenyl-cyclase pathway.
Low-dose radiation therapy (i.e., therapeutic radiation hormesis) has already been reported to be successful for some types of cancer (Chaffey et al. 1976; Choi et al. 1979; Sakamota et al. 1997; Richaud et al. 1998; Cuttler et al. 2000; Cuttler and Pollycove 2003; Sakamota 2004; Kaminski et al. 2005). Fractionated, low-dose, total-body, and half-body external beam therapy has been used successfully by several medical groups in treating non-Hodgkin's lymphoma (Chaffey et al. 1976; Choi et al. 1979; Richaud et al. 1998; Cuttler et al. 2000, Cuttler and Pollycove 2003; Sakamota 2004). Small individual doses (called fractions) are administered after designated time intervals over a given time period. Dose fraction sizes used in treating non-Hodgkin's lymphoma have been relatively large, e.g., 100 to 150 mGy (Cuttler et al. 2000). Our research results indicate that much smaller fraction sizes may be equally effective and, if so, would allow for considerable extension of the total period over which dose fractions were given.
Therapeutic radiation hormesis has also been successfully employed to treat ovarian, colon, and hematologic cancer, with no symptomatic side effects (Cuttler and Pollycove 2003; Sakamoto 2004). Low-dose, low-dose-rate radioimmunotherapy (a form of radiation hormesis involving beta radiation) has also been used successfully in treating follicular lymphoma (Kaminski et al. 2005).
The PAM process is expected to be more efficiently activated by low-dose-rate and fractionated exposures than by high-dose-rate and single exposures. The time interval between the dose fractions could be quite critical. For new research, biweekly or once monthly fractions could be initially investigated. The number of fractions could be large without serious side effects, so long as small fraction sizes (e.g., 0.5 to 1 mGy) were used.
ENVIRONMENTAL RADIATION HORMESIS
Numerous studies have demonstrated that environmental exposures to ionizing radiation can suppress cancer and other diseases (environmental radiation hormesis). Indeed, immune responses have been found to be upregulated among inhabitants of high natural background radiation areas (Luckey 1991; Safwat 2000; Kojima et al. 2002). Table 4 shows PROFAC estimates for environmental radiation hormesis associated with exposure to elevated levels of radon. The PROFAC estimates are based on SMR values reported by Mifune et al. (1992) based on a study of cancer deaths (1952–1991) for persons residing in a high-level radon spa area of Japan. The SMR was evaluated relative to the Japanese population. The results in Table 4 suggest that many lives are being saved worldwide due to environmental radiation hormesis (e.g., associated with radon exposure in the home). The results also support the use of radon in the treatment and prevention of genomic-instability-associated diseases. Immune system stimulation from radon exposure may also be protecting us from diseases not associated with genomic instability. Eliminating radon from the home (often costly for homeowners) therefore may be causing an increased risk of diseases.
TABLE 4.
Central estimates of high-level, radon-associated PROFACs against cancer at different sites in the body based on cancer mortality data for persons residing in a high-level radon spa area in Japan (Mifune et al. 1992)
| PROFACa | ||
|---|---|---|
| Cancer site or type | Females | Males |
| Leukemia | 0.47 | 0.56 |
| Stomach | 0.55 | 0.60 |
| Breast | 0.74 | - |
| Lung | 0.81 | 0.53 |
| Colon/rectum | 0.86 | 0.70 |
Presumed to be associated with environmental radiation hormesis.
With our HRR model, one can calculate the expected impact of reducing natural background ionizing radiation to zero. For such calculations, it is convenient to use normalized dose DL/b where b is any reference background low-LET radiation dose over the period of interest. Our previous research has revealed that only DL (the low-LET dose) is important for evaluating cancer risk at below natural background radiation exposures (Scott 2006b). This is because thresholds for activating adapted protection depend on DL but not on DH. Changes in risk at below natural radiation levels is modeled via the HRR model as being related to loss of adapted protection. Each individual has a different threshold dose (stochastic) DL for activating the system of protective processes discussed. Assuming the indicated stochastic thresholds to be uniformly distributed over the interval 0 (absolute zero natural background radiation) to the dose D* (which exceeds background radiation dose O+) in Figure 3, the RR can be characterized by the following linear relationship:
| (3) |
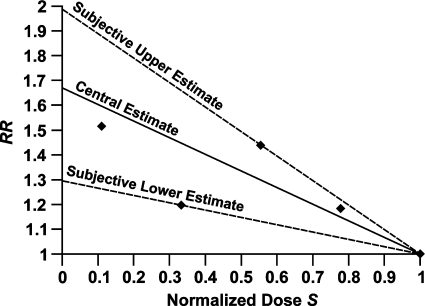
where RR* is the relative risk at absolute zero natural ionizing radiation dose and S = DL/b is the normalized dose relative to an arbitrarily assigned reference background radiation dose b. RR takes on a value of 1 at S = 1, which correspond to the absorbed dose DL = b. Figure 8 shows results of applying Equation 3 to environmental radiation hormesis data for solid cancer mortality in Yangjiang, China based on data for the years 1979–1998 reported by Wei and Sugahara (2002). Normalized doses were evaluated relative to a cumulative dose of 450 mSv. Here it was assumed that the low-LET component of the dose was proportional to the total dose in millisieverts. Thus, the normalized dose based on millisiev-erts estimates the normalized dose based on DL. Three curves are presented in Figure 8: central, lower, and upper bounds. The central curve is based on constrained linear regression. Straight lines were drawn from each data point through the coordinates (RR = 1, S = 1). The average of these lines was used for the central curve. The upper and lower bound curves are subjective and are based on the curves with the steepest and shallowest slopes. RR is projected to increase between 1.3- and 2.0-fold as background radiation is reduced to absolute zero.
FIGURE 8.
Expected effects of reducing natural background radiation on cancer mortality, based on solid cancer mortality data for Yangjiang, China, reported by Wei and Sugahara (2002). Central (from constrained linear regression), and subjective upper and lower bound curves are presented. Normalized doses S were evaluated relative to a total dose of 450 mSv.
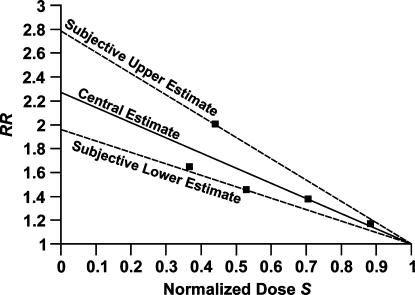
Similar results are shown in Figure 9 for cancer among inhabitants of various cites and states in India, based on data from an ecological study conducted by Nambi and Soman (1987). Normalized dose was evaluated relative to an annual gamma-ray dose of 850 μGy (a relatively large dose) from natural background radiation. Thus, only gamma-ray doses were used. Cancer RR is projected to increase 2.0- to 2.8-fold as natural background radiation is reduced to absolute zero.
FIGURE 9.
Expected effects of reducing natural background radiation on cancer relative risk based on data for various cities and states of India reported by Nambi and Soman (1987). Central (from constrained linear regression), and subjective upper and lower bound curves are presented. Normalized doses S were evaluated relative to an annual gamma-ray dose of 850 μSv.
For calculations associated with Figures 8 and 9, it was assumed that high-fidelity DNA repair is not lost at near absolute zero background radiation, which may not be the case. In vivo mutation data of Hooker et al. (2004) that we have previously modeled indicated the loss of high-fidelity DNA repair after ultra low X-ray doses (Scott, 2005a; Scott et al. 2006) when cells from irradiated mice were frozen shortly (3 hours) after irradiation (not allowing for background radiation to build to a level which would trigger protective processes). Mutation risk increases by orders of magnitude apparently due to the loss of high-fidelity DNA repair along with loss of the PAM process. Thus, the increases indicated in Figures 8 and 9 as S approaches 0 (absolute zero ionizing radiation dose) may be greatly underestimated. Background low-LET ionizing radiation may be essential for making high-fidelity DNA repair available on a regular basis to all mammalian life. The possibility that high-fidelity DNA repair may be significantly less available after low radiation doses has been proposed by others based on experimental measurements of DNA double-strand break repair using a sensitive assay (Rothkamm and Löbrich 2003). Further, in vitro data for gamma-ray induced chromosomal aberrations in human lymphocytes suggest differing fidelity for DNA repair after low and moderate doses delivered at low or high rates (Zaichkina et al. 2004). Low-fidelity repair was implicated for doses < 200 mGy and a much higher fidelity repair for doses between 200 and 500 mGy.
CONCLUSIONS
Environmental radiation hormesis associated with radon in our homes and with elevated background radiation (low- or low- plus high-LET) appears to be preventing many cancer deaths.
Medical radiation hormesis associated with routine applications of diagnostic chest X-rays, mammograms, and CT scans may be preventing cancer occurrence through stimulating the removal of precancer-ous neoplastically transformed cells. Medical and environmental radiation hormesis may also be preventing metastasis of existing cancer.
Low-dose therapeutic radiation hormesis associated with fractionated exposure to small X-ray doses has been used to successfully treat non-Hodgkin's lymphoma and ovarian, colon, and hematologic cancer.
Low-dose, low-dose-rate therapeutic radiation hormesis associated with application of radiolabeled antibodies (beta radiation source) has been used successfully to treat follicular lymphoma.
Low-dose therapeutic radiation hormesis in combination with apopto-sis-sensitizing agents such as resveratrol could be used to successfully cure cancer.
ACKNOWLEDGMENTS
This research was supported by the Office of Science (BER), U.S. Department of Energy (DOE) Grants DE-FG02-03ER63671 and DE-FG02-03ER63657. We are grateful to Vicki Fisher and Cynthia Herrera for editorial assistance. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsement, either express or implied, of the DOE or of Lovelace Respiratory Research Institute.
REFERENCES
- Aggarwal BB, Bhardwau A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:3–60. [PubMed] [Google Scholar]
- Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar K. Resveratrol causes WAF-1/p21-mediated G1-arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897-1997. Br J Radiol. 2001;74:507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- Boice JD, Morin MM, Glass A, Friedman GD, Stovall M, Hoover RN, Fraumeni JF. Diagnostic x-ray procedures and risk of leukemia, lymphoma, and multiple myeloma. JAMA. 1991;265(10):1290–1294. [PubMed] [Google Scholar]
- Bonner WM. Low-dose radiation: Thresholds, bystander effects, and adaptive response. PNAS. 2003;100(9):4973–4975. doi: 10.1073/pnas.1031538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey JT, Rosenthal DS, Moloney WD, Hellman S. Total body irradiation as treatment for lym-phosarcoma. Int J Radiat Oncol Biol Phys. 1976;1:399–405. doi: 10.1016/0360-3016(76)90004-3. [DOI] [PubMed] [Google Scholar]
- Chen WL, Luan YC, Shieh MC, Chen ST, Kung HT, Soong KL, Yeh YC, Chou TS, Mong SH, Wu JT, Sun CP, Deng WP, Wu MF, Shen ML. Is chronic radiation an effective prophylaxis against cancer? J Amer Phsy Surg. 2004;9(1):6–10. [Google Scholar]
- Choi NC, Tinothy AR, Kaufman SD, Carey RW, Aisenbert AC. Low-dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin's lymphoma. Cancer. 1979;43:1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventative agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- Cohen BL. Test of the linear-no-threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68(2):157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cuttler JM, Pollycove M, Welsh JS. Application of low doses of radiation for curing cancer. CNS Bulletin. 2000;21(2):45. [Google Scholar]
- Cuttler JM, Pollycove M. Can cancer be treated with low doses of radiation? J Amer Phys Surg. 2003;8(4):108–111. [Google Scholar]
- Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–4739. [PubMed] [Google Scholar]
- Elmore E, Lao XY, Ko M, Rightnar S, Nelson G, Redpath J. Neoplastic transformation in vitro induced by low doses of 232 MeV protons. Int J Radiat Biol. 2005;81(4):291–297. doi: 10.1080/09553000500140324. [DOI] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Sondhaus CA. Responses to low doses of ionizing radiation in biological systems. Nonlin Biol Toxicol Med. 2004;2(3):143–171. doi: 10.1080/15401420490507431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Festa F, Cornetta T, Ricordy R, Cozzi R. Resveratrol affects X-ray induced apoptosis and cell cycle delay in human cells in vitro. In J Mol Med. 2005;15:1005–1012. [PubMed] [Google Scholar]
- Frigèrio NA, Stowe RS. 1976. Carcinogenic and genetic hazard from background radiation. Proceedings of Biological Effects of Low-Level Radiation Pertinent to Protection of Man and His Environment, Chicago, November 3-7, 1975, Volume II. Vienna, pp. 385-393. International Atomic Agency
- Garg AK, Buchholz TA, Bharat B, Aggarwal B. Forum Review. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antiox Redox Signa. 2005;7(11,12):1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- Gilbert ES, Cragel DL, Wiggs LD. Updated analyses of combined mortality data of workers at the Hanford Site, Oak Ridge National Laboratory, and Rocky Flats Weapons Plant. Radiat Res. 1993;136:408–421. [PubMed] [Google Scholar]
- Gribbin MA, Howe GR, Weeks JL. A Study of the Mortality of AECL Employees. V. 1992. The Second Analysis: Mortality during the Period 1950-1985. Atomic Energy of Canada Limited; Report AECL-10615
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matsushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- Hooker AM, Bhat M, Day TK, Lane JM, Swinburne SJ, Morley AA, Sykes PJ. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res. 2004;162:447–452. doi: 10.1667/rr3228. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Sakamoto K. Suppressive effect of low-dose total body irradiation on lung cancer metastases: dose dependence and effective period. Radiother Oncol. 1993;26(2):177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Howe GR. Lung cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with lung cancer mortality in the atomic bomb survivors study. Radiat Res. 1995;142:295–304. [PubMed] [Google Scholar]
- Hsieh T, Wu JM. Differential effects on growth, cell cycle arrest and induction of apoptosis by resveratrol in human prostate cancer cell line. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Gorski AI, Maksioutov MA, Tsyb AF, Souchkevitch GN. Mortality among the Chernobyl emergency workers: estimation of radiation risks (preliminary analysis) Health Phys. 2001;81(5):514–521. doi: 10.1097/00004032-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Jaworowski Z. 2001. Ionizing radiation in the 20th century and beyond. Symposium “Entwicklungen im Strahleschutz”. Munich, November 29. Available at: http://www.cns-snc.ca/branches/Toronto/radiation/
- Joiner MC, Lambin P, Marples B. Adaptive response and induced resistance. CR Acad Sci III. 1999;322:167–175. doi: 10.1016/s0764-4469(99)80040-7. [DOI] [PubMed] [Google Scholar]
- Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, Regan D, Kison P, Fisher S, Kroll S, Wahl RL. 131I-Tositumomab as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5):441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- Kauffman J. Diagnostic radiation: are the risks exaggerated? J Amer Phys Surg. 2003;8(2):54–55. [Google Scholar]
- Khokhryakov VF, Menshikh ZS, Migurova NI. Problems of the occurrence of pneumosclerosis and lung cancer among workers exposed by inhalation to plutonium aerosols. Radiat Safety. 1996;2:51–55. in Russian. [Google Scholar]
- Ko SJ, Liao X-Y, Molloi S, Elmore E, Redpath JL. Neoplastic transformation in vitro after exposure to low doses of mammographic-energy X rays: Quantitative and mechanistic aspects. Radiat Res. 2004;162:646–654. doi: 10.1667/rr3277. [DOI] [PubMed] [Google Scholar]
- Kojima S, Ishida H, Takahasji M, Yamaoka K. Elevation of gluthatione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res. 2002;157:275–280. doi: 10.1667/0033-7587(2002)157[0275:eogibl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Boca Raton, Florida: CRC Press; 1991. Radiation Hormesis. [Google Scholar]
- Liu S-Z, Liu WH, Sun JB. Radiation hormesis: its expression in the immune system. Health Phys. 1987;52:579–583. doi: 10.1097/00004032-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Liu S-Z, Su X, Zhang YC, Zhao Y. Signal transduction in lymphocytes after low doses of radiation. Int J Occup Med Toxicol. 1994;3:107–117. [PubMed] [Google Scholar]
- Liu S-Z. Nonlinear dose-response relationship in the immune system following exposure ionizing radiation: Mechanisms and implications. Nonlin Biol Toxicol Med. 2003;1(1):71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-Z. Radiation-induced change in lymphocyte proliferation and its neuroendocrine regulation: Dose-response relationship and pathophysiological implications. Nonlin Biol Toxicol Med. 2004;2(3):233–244. doi: 10.1080/15401420490507486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T, James SJ. T cell potentiation by low dose ionizing radiation: possible mechanisms. Health Phys. 1990;59(1):29–34. doi: 10.1097/00004032-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Mifune M, Sobue T, Arimoto H, Komoto Y, Kondo S, Tanooka H. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn J Cancer Res. 1992;83:1–5. doi: 10.1111/j.1349-7006.1992.tb02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ, Jackson JS, Morrison DP, Carlisle SM. Low doses and of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer prone, radiation sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchel REJ. The bystander effect: recent developments and implications for understanding the dose response. Nonlin Biol Tox Med. 2004;2(3):173–183. doi: 10.1080/15401420490507512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ. Radiation risk prediction and genetics: the influence of the TP53 gene in vivo. Dose Response. 2005;3:519–532. doi: 10.2203/dose-response.003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambi KSV, Soman SD. Environmental radiation and cancer in India. Health Phys. 1987;52:653–657. doi: 10.1097/00004032-198705000-00018. [DOI] [PubMed] [Google Scholar]
- NCRP (National Council on Radiation Protection and Measurements). 1997. Ionizing Radiation Exposure of the Population of the United States. NCRP Report 93, Bethesda, MD
- NRC (National Research Council). 2006. Health Risks from Exposure to Low Levels of Ionizing Radiation. The National Academies Press; Report BEIR-VII, Phase 2. Available at: www.nap.edu [PubMed]
- Nyström L, Andersson I, Bjurstam N, Freisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mamography screening: updated overview of the Swedish randomised trials. The Lancet. 2002;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Possible effect of inducible protective responses in mitigating endogenous damage. Hum Exper Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, James C, Christie E, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Lu Q, Lao X, Molloi S, Elmore E. Low doses of diagnostic energy X-rays protect against neoplastic transformation in vitro. Int J Radiat Biol. 2003;79(4):235–240. doi: 10.1080/0955300031000096306. [DOI] [PubMed] [Google Scholar]
- Redpath JL. Nonlinear response for neoplastic transformation following low doses of low LET radiation. Nonlin Biol Tox Med. 2005;3:113–124. doi: 10.2201/nonlin.003.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richaud PM, Soubeyran P, Eghbali H, Chacon B, Marit G, Broustet A, Hoerni B. Place of low-dose total body irradiation in treatment of localized follicular non-Hodgkin's lymphoma: results of a pilot study. Int J Radiat Oncol Biol Phys. 1998;40:387–390. doi: 10.1016/s0360-3016(97)00722-0. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. PNAS. 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HH, Zaider M. Radiogenic lung cancer: the effects of low doses of low linear energy transfer (LET) radiation. Radiat Environ Biophys. 1997;36:85–88. [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, Oda T, Iwasaki T, Fujita K, Yamada T, Tanooka H. Suppression of carcinogenic process in mice by chronic low dose rate gamma-irradiation. Int J Low Radiat. 2003;1(1):142–146. [Google Scholar]
- Safwat A. The immunobiology of low-dose total-body irradiation: more questions than answers. Radiat Res. 2000;153:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Myojin M, Hosoi Y, Ogawa Y, Nemoto K, Takai Y, Kakuto Y, Yamada S, Watabe M. Fundamental and clinical studies on cancer control with total or upper-half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9:161–175. [Google Scholar]
- Sakamoto K. Radiobiological basis for cancer therapy by total or upper-half body irradiation. Nonlin Biol Toxicol Med. 2004;2(4):293–316. doi: 10.1080/15401420490900254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. A biological-based model that links genomic instability, bystander effects, and adaptive response. Mutat Res. 2004;568:129–143. doi: 10.1016/j.mrfmmm.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Scott BR, Walker DM, Walker VE. Low-dose radiation and genotoxic chemicals can protect against stochastic biological effects. Nonlinearity. 2004;2:185–211. doi: 10.1080/15401420490507602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Stochastic thresholds: a novel explanation of nonlinear dose-response relationships for stochastic radiobiological effects. Dose-Response. 2005a;3(4):547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. 2005b. Low-dose radiation risk extrapolation fallacy associated with the linear-no-threshold model. BELLE Newsletter 13(2) Part 2; December 2005b:22-27 [DOI] [PubMed]
- Scott BR. 2006a. Low-dose radiation-induced protective process and implications for risk assessment, cancer prevention, and cancer therapy. Dose-Response (in press) [DOI] [PMC free article] [PubMed]
- Scott BR. 2006b. Radiation hormesis and the control of genomic instability. In: New Research on Genomic Instability. Nova Sciences Publishers, Inc. Hauppage, NY; (accepted)
- Scott BR, Haque M, Di Palma J. 2006. Biological basis for radiation hormesis in mammalian cellular communities. Int J Low Radiat (in press)
- Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittandini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res. 2000;496:171–180. doi: 10.1016/s1383-5718(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. PNAS. 2003;100(19):10671–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinhofer I, Bernhard D, Senfter M, Anether G, Loeffler M, Kroemer G, Kofler R, Csordas A, Greil R. Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl-2. FASEB J. 2001;15:1613–1615. doi: 10.1096/fj.00-0675fje. [DOI] [PubMed] [Google Scholar]
- Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: The joint report of The Académie des Sciences (Paris) and of The Académie Nationale de Médicine. Int J Radiat Oncol Biol Phys. 2005;63(2):317–319. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tubiana M, Aurengo A, Averbeck D, Bonin A, Le Guen B. Masse R, Monier R, Valleron A-J, and de Vathaire F. 2005. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionizing radiation. Académie des Sciences Report March 30, 2005, Nat Acad Med (France). Académie Nationale de Médicine report
- Ullrich RL, Jernigan MC, Cosgrove GE, Satterfield LC, Bowles ND, Storer JB. The influence of dose and dose rate on the incidence of neoplastic disease in RFM mice after neutron irradiation. Radiat Res. 1976;68(1):115–131. [PubMed] [Google Scholar]
- Ullrich RL, Storer JB. Influence of γ irradiation on the development of neoplastic disease in mice. II. Solid tumors. Radiat Res. 1979;80:317–324. [PubMed] [Google Scholar]
- Wang G-J, Li X-K, Sakai K, Cai L. Low-dose radiation and its clinical implications: diabetes. BELLE Newsletter. 2005;September 13(2):12–21. doi: 10.1177/0960327108090752. Part 1. [DOI] [PubMed] [Google Scholar]
- Wei L-X, Sugahara T. Recent advances of “epidemiological study in high background radiation area in Yangjiang, China.”. International Congress Series. 2002;1236:91–99. [Google Scholar]
- Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiation become refractory to high doses of radiation as well as to chemical muta-gens that induced double-strand breaks in DNA. Int J Radiat Biol. 1988;53(1):39–48. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- Zaichkina SI, Rozanova OM, Aptikaeva GF, Achmadieva ACh, Klokov DY. Low doses of gamma-radiation induced nonlinear dose responses in mammalian and plant cells. Nonlin Biol Tox Med. 2004;2(3):213–221. doi: 10.1080/15401420490519861. [DOI] [PMC free article] [PubMed] [Google Scholar]