Abstract
Antimutagenic DNA damage-control is the central component of the homeostatic control essential for survival. Over eons of time, this complex DNA damage-control system evolved to control the vast number of DNA alterations produced by reactive oxygen species (ROS), generated principally by leakage of free radicals from mitochondrial metabolism of oxygen. Aging, mortality and cancer mortality are generally accepted to be associated with stem cell accumulation of permanent alterations of DNA, i.e., the accumulation of mutations. In a young adult, living in a low LET background of 0.1 cGy/y, the antimutagenic system of prevention, repair and removal of DNA alterations reduces about one million DNA alterations/cell/d to about one mutation/cell/d. DNA alterations from background radiation produce about one additional mutation per 10 million cells/d. As mutations accumulate and gradually degrade the antimutagenic system, aging progresses at an increasing rate, mortality increases correspondingly, and cancer increases at about the fourth power of age. During the past three decades, genomic, cellular, animal and human data have shown that low-dose ionizing radiation, including acute doses up to 30 cGy, stimulates each component of the homeostatic antimutagenic control system of antioxidant prevention, enzymatic repair, and immunologic and apoptotic removal of DNA alterations. On the other hand, high-dose ionizing radiation suppresses each of these antimutagenic protective components. Populations living in high background radiation areas and nuclear workers with increased radiation exposure show lower mortality and decreased cancer mortality than the corresponding populations living in low background radiation areas and nuclear workers without increased radiation exposure. Both studies of cancer in animals and clinical trials of patients with cancer also show, with high statistical confidence, the beneficial effects of low-dose radiation.
I. INTRODUCTION
Four decades of genomic, cellular, animal and human data have shown that low-dose ionizing radiation stimulates positive genomic and cellular responses associated with effective cancer prevention and therapy and increases the life span of mammals and humans.[1–8] Nevertheless, this data is questioned because it seems to contradict the unquestioned linear relation between ionizing radiation dose and damage to DNA without providing a clear mechanistic explanation of how low-dose radiation could produce such beneficial effects. Acknowledgment of the validity of this contradictory data would destroy the basis of a very expensive system of regulation and remediation.
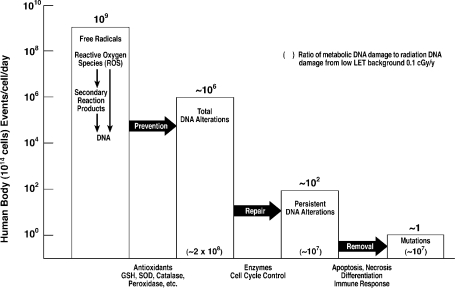
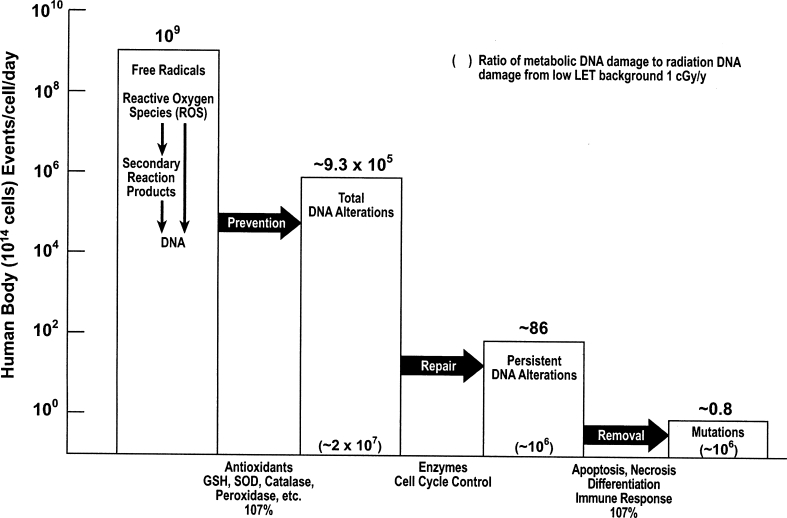
A quantitative understanding of the antimutagenic DNA damage-control system essential for survival was recently developed[9] and is illustrated in Figure 1. This complex system evolved in aerobic organisms over eons of time in order to control an enormous, relentless burden of DNA alterations produced by reactive oxygen species (ROS), generated principally by free radicals leaked from mitochondrial oxygen metabolism. This antimutagenic system also operates against the DNA damage generated by ionizing radiation ROS and by chemicals. The enhanced response of the antimutagenic system to low-dose radiation provides a clear mechanistic explanation of the beneficial effects seen in cells, mammals and humans.
FIGURE 1.
The antimutagenic DNA damage-control biosystem. Estimates are based on data in the literature.9
II. THE ANTIMUTAGENIC DNA DAMAGE-CONTROL SYSTEM
The immune system is an essential component of antimutagenic control of cumulative DNA damage and metabolic damage generated by a relentless burden of DNA alterations produced by ROS leaked from mitochondria.[10] In addition to removal of persistent DNA alterations by the immune system and cellular programmed self-destruction (apoptosis), the human antimutagenic system includes antioxidant prevention and enzymatic repair of DNA damage. This complex biosystem of prevention, repair and removal sequentially reduces DNA damage from about one million DNA alterations/cell/day to about one “mutation”/cell/day (Figure 1). In contrast, low LET background radiation of 1 mGy/year produces 1 DNA alteration/500 cells/day. Double-strand breaks/cell/day generated by oxygen metabolism is 1000 times greater than the double-strand breaks produced by this background radiation. The UNSCEAR 1994 Report[11] and recent studies[12, 13] furnish extensive documentation of low-dose stimulation of many cellular functions including: antioxidant prevention (Figure 2)[14], enzymatic repair (Figures 3 and 4)[15, 16], and immunologic and apoptotic removal (Figure 5)[17] of DNA damage. This stimulation of each of these antimutagenic responses by low-dose radiation, in contrast to their suppression by high-dose radiation, predictably precludes a linear dose-response relation of radiation and health effects.[18] Enhanced prevention of gene mutations by increased low-dose radiation (Figure 6) is associated with decreased mortality and decreased cancer mortality observed in human populations exposed to low-dose radiation.[19–21] Stimulation of the immune system by low-dose radiation prevents and removes cancer metastases in rodents and humans.
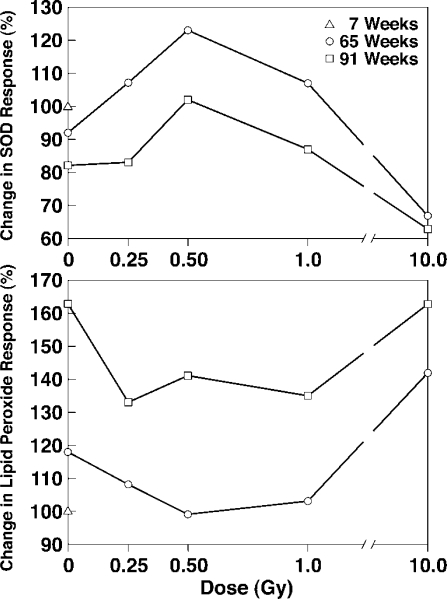
FIGURE 2.
Antioxidant SOD and lipid peroxide response to age and radiation of rat brain cortex14
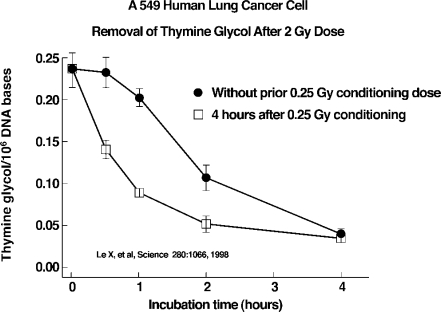
FIGURE 3.
Low dose induced DNA repair15
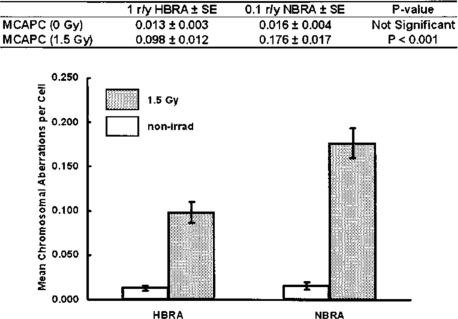
FIGURE 4.
Mean chromosomal aberrations per cell in lymphocytes before and after exposure to 150 r. Lymphocytes were obtained from Ramsar residents in a high background γ radiation area of about 10 mGy/y and residents in a normal background γ radiation area of about 1 mGy/y.16
FIGURE 5.
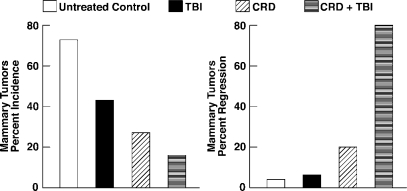
Eight month old, mammary tumor-susceptible, female C3H/He mice were first adjusted in a stepwise manner to chronically restricted diet (calorically 70% of ad libitum diet) over a period of 3 weeks. The mice were maintained on CRD until completion of the study. After their diet was adjusted, the mice were exposed to TBI (0.04 Gy, 3 alternating days/week, 4 weeks) and were observed for 35 weeks. Tumor regression of the CRD + TBI group was very rapid and large numbers of CD8+ T cells were found infiltrating the regressing tumors, which were not seen in mice of the untreated control, LDR and CRD groups.17
FIGURE 6.
The antimutagenic DNA damage-control biosystem response to high background radiation = 120%. Estimates based on data in the literature.9
III. IMMUNE SYSTEM RESPONSE TO RADIATION
Low-dose total body irradiation (TBI) and chronic TBI (LDR) stimulate immune system prevention and removal of cancer metastases. This has been observed in mice for about 40 years[16, 22, 23] and more recently in rats[24] and humans.[3–6, 8, 25–29]
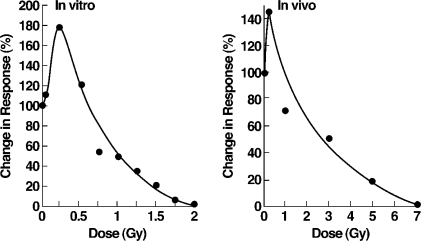
The maximal immune response of mouse spleen T lymphocytes to sheep red blood cells, both in vitro and in vivo, occurs after a single dose of 0.25 Gy or 25 r (Figure 7).[23] The maximal in vitro response is 180% with suppression to 50% of control after 100 r. The maximal in vivo response is 145%, but more than 260 r is needed for suppression to 50% of control.
FIGURE 7.
Immune system response to radiation. Mouse splenic cells primed with antigenic sheep red blood cells.23
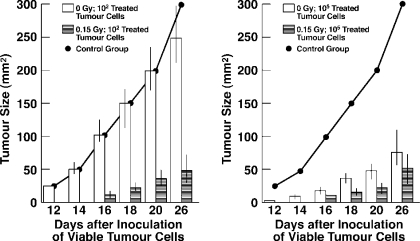
TBI given with subimmunogenic tumor antigen induces tumor immunization. Subcutaneous inoculation of sham irradiated controls with 100 non-viable tumor cells does not suppress growth of 10,000 viable tumor cells inoculated subcutaneously 21 days later. Strikingly, 15 r of TBI given simultaneously with inoculation of 100 non-viable tumor cells does induce marked suppression of tumor cell growth, exceeding that induced by 100,000 non-viable tumor cells without TBI (Figure 8).[22]
FIGURE 8.
Effect of 0.15 Gy upon response of A/J mice to subimmuno-genic and immunogenic numbers of non-viable mitomycin-treated fibrosarcoma (SaI) tumor cells. Groups of 60 mice were exposed to whole-body irradiation or sham-irradiated and inoculated subcutaneously with the indicated numbers of mitomycin-treated tumor cells. Twenty-one days later, all animals received 104 untreated SaI cells and were followed for tumor size. A control group did not receive mitomycin-treated cells.22
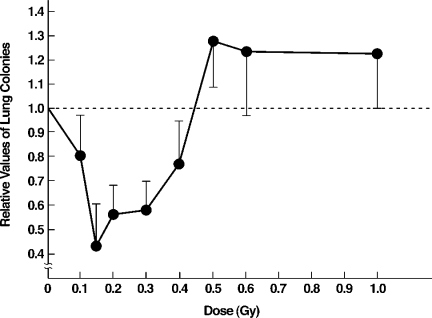
TBI stimulates immune suppression of tumor metastases to the lung (Figure 9).[8] Lung colonies, counted 20 days after TBI given 12 days after tumor cell transplantation into the axilla of mice, were decreased by TBI doses less than 50 r; 15 r induced the maximal decrease of 60%. However, high doses in the 50–100 r range suppressed the immune system, with increased metastases to lung.
FIGURE 9.
TBI given 12 days after tumor cell transplantation into axilla. Lung colonies counted 20 days after TBI. Low dose TBI ineffective with spleen blocked. Low dose splenic irradiation, half-body irradiation (HBI) and TBI equally effective.8
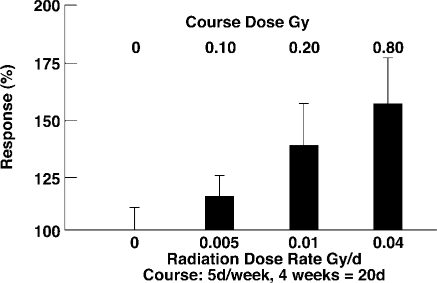
Chronic TBI (LDR) stimulates immune response of spleen T lymphocyte proliferation in mice (Figure 10).[23] Mice irradiated 5 days/week for 4 weeks with LDR courses of 10 r (0.5 r/d), 20 r (1.0 r/d) and 80 r (4.0 r/d) showed lymphocyte responses of 115%, 140%, and 160%, respectively, relative to 100% proliferation in the unirradiated control group.
FIGURE 10.
Dose-response analysis of splenic T cell proliferative response 3–5 days after the last radiation exposure of immunologically normal, long-lived C57B1/6J+/+ mice. Results are expressed as the mean percent increase in 3H-thymidine uptake relative to 0 Gy control group as 100%. The vertical bars = 1 SEM.23
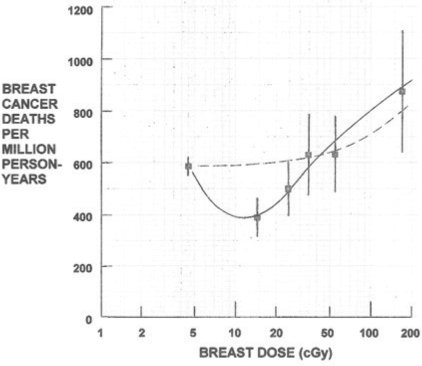
LDR with a calorically-restricted diet, of 70% ad libitum diet calories, prevents and removes spontaneous breast cancer tumors in mice (Figure 5).[17] Eight-month-old breast tumor susceptible female mice, after 3-week adjustment to CRD, were exposed to a 48 r, 4-week course of LDR (4 r 3d/week) and then observed for 35 weeks. While 73% of the ad libitum diet mice and 27% of the CRD mice developed breast cancer, only 16% of CRD + LDR mice developed breast cancer. Most impressive was the very rapid 80% tumor regression of CRD + LDR mice compared to the 20% and 4% regression in CRD and control mice, respectively. Large numbers of “killer” cytotoxic CD8+ T lymphocytes were observed infiltrating regressing tumors of CRD+LDR mice, but not in the control and CRD mice. Half-body LDR of women given 5–30 r by 25 to 150 fluoroscopic lung examinations similarly decreased breast cancer mortality. Breast cancer mortality of those receiving doses between 10–20 r was reduced to 66% of controls without LDR (Figure 11).[24,25]
FIGURE 11.
Reduced breast cancer mortality of tuberculosis patients who received LDI during fluoroscopy24, 25
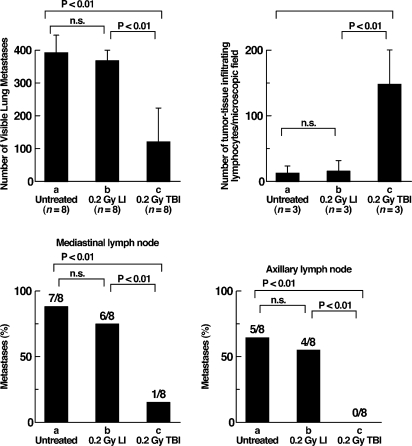
Metastasis is also suppressed by TBI of tumor-bearing rats (Figure 12).[26] TBI or irradiation localized to tumor implanted into the leg or control sham-irradiation were given 14 days after tumor implantation. The number of visible metastases in the lung and the incidence of metastases in mediastinal and axillary lymph nodes were obtained 50 days after implantation. The number of tumor infiltrating lymphocytes/microscopic field was observed 21 days after implantation. Metastases to the lung, mediastinum and axillary lymph nodes in TBI rats were reduced by more than 70% of that in control and locally irradiated rats. Tumor infiltration by lymphocytes in TBI rats was more than 900% of that in control and locally irradiated rats. Cytotoxic CD8+ T lymphocytes in the spleen of TBI rats were increased to 176% of those in control and locally irradiated rats.
FIGURE 12.
The number and incidence of metastases in lung and lymph nodes of mediastinum and axilla 50 days after intramuscular (leg) tumor implantation in rats, and the number of tumor infiltrating lymphocytes 21 days after implantation. Total body or localized tumor irradiation with 0.2 Gy was given 14 days after implantation of 5 × 105 allogenic hepatoma cells.26
IV. HUMAN LOW DOSE RADIATION (LDR) CANCER IMMUNOTHERAPY
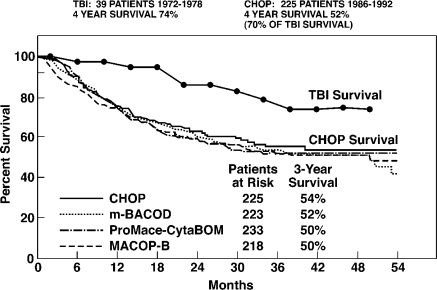
Two Harvard University clinical trials of LDR therapy in patients with non-Hodgkin's lymphoma were published in 1976[27] and in 1979 (Figure 13).[28] The protocols were very similar. The Chaffey, et al. 1976 trial used a 150 r LDR course with TBI doses of 15 r 2x/week for 5 weeks. The Choi, et al. 1979 trial also used a 150 r LDR course with TBI doses of either 15 r 2x/week or 10 r 3x/week for 5 weeks. In both studies transient low platelets requiring interruption of scheduled therapy occurred in 35–40% of patients, irrespective of 10 r or 15 r dose schedule. Both chemotherapy and LDR patients had previously received chemotherapy and localized tumor high-dose radiation. Histologic tumor grades of LDR and chemotherapy patients were similar. COP chemotherapy used in the 1976 trial was replaced by the more effective CHOP chemotherapy still in current use. Both trials furnish 4-year survival data. Four-year survival in the 1976 study of 25 LDR patients is 70% compared with 40% survival of 24 matched patients treated with COP.[26] The 1979 trial shows a similar 74% survival of 39 LDR patients compared with improved 52% survival of 225 patients treated with CHOP (Figure 13).[28]
FIGURE 13.
Comparison of TBI with CHOP chemotherapy. CHOP remains the best available chemotherapy treatment for patients with advanced-stage intermediate-grade or high-grade non-Hodgkin's lymphoma.28
Sakamoto, et al., Tohoku University, Sendai, Japan, published a 1997 review of their experimental studies in mice and a clinical trial of LDR. In mice, 15 r TBI induced maximal suppression of tumor metastasis (Figure 9).[8] TBI given 6–12 hours before localized high-dose tumor therapy increases the effectiveness of tumor therapy. TBI, upper half body irradiation (HBI), and localized irradiation of the spleen were equally effective in stimulating the immune system of mice.
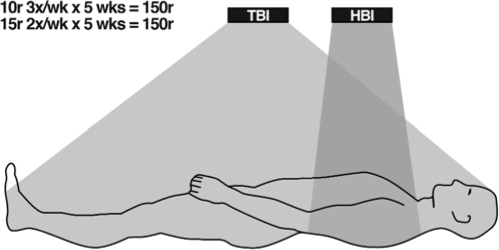
The protocol used by Sakamoto, et al. in their clinical trial of LDR therapy of patients with non-Hodgkin's lymphoma is similar to that used by Choi, et al. Both used a 150 r LDR course with equally effective TBI doses of either 15 r 2x/week or 10 r 3x/week for 5 weeks in patients with previous CHOP chemotherapy and localized high-dose tumor irradiation. Choi, et al. used TBI, while Sakamoto, et al. used TBI or HBI (Figure 14) with equal effectiveness without interruption of scheduled therapy by low platelets.
FIGURE 14.
Treatment of patients with non-Hodgkin's lymphoma with half (HBI) or total (TBI) body irradiation. Adapted from Sakamoto et al8
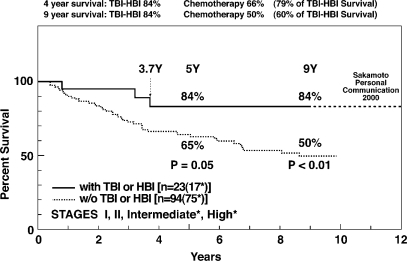
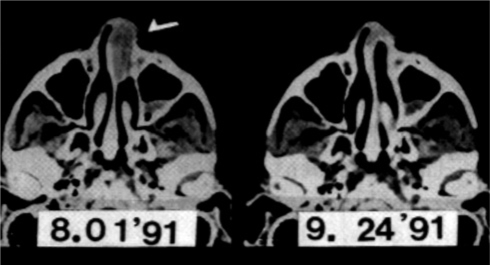
Sakamoto, et al. report 9-year survival of 23 LDR patients and 94 CHOP chemotherapy patients with similar histologic tumor grades, approximately 75% of each group having intermediate or high grade lymphoma (Figure 15).[8] Tumors outside the HBI field regressed completely in response to LDR (Figure 16).[29] Nine-year survival of patients treated with LDR is 84%, unchanged from their 31/2-year survival. Survival of these LDR patients at 12 years remains 84% (personal communication). In comparison, the 9-year survival of CHOP chemotherapy patients is 50% (Figure 15).[8]
FIGURE 15.
Utility of low-dose irradiation of HBI or TBI for patients with non-Hodgkin's lymphoma. Patients in both groups received chemotherapy and localized tumor high-dose radiation.8
FIGURE 16.
CT scans of upper nasal cavity before and after HBI therapy. Though entirely outside the HBI field, the nasal tumor completely disappeared.29
Comparison of 4-year survival in the Harvard and Tohoku LDR vs CHOP trials are consistent in both showing about a 20% better survival of LDR patients compared with CHOP patients. In the Japanese trial, however, moderate decreases of platelets did not require schedule interruption, and the 4-year survival of both LDR and CHOP patients was increased about 10% above those of the United States trial. This may be related to the well-established benefits of lower caloric intake and more exercise in the Japanese population. Though racial differences may be a factor, this has not been demonstrated in Japanese living in the United States. As shown by Makinodan (Figure 5)[17], LDR therapy is more effective when administered to mice with optimal caloric intake and better initial immune system activity.
V. NEED FOR CLINICAL TRIALS OF LDR IMMUNOTHERAPY OF BREAST, PROSTATE AND COLORECTAL CANCER
Despite many hundreds of clinical trials of chemotherapy during the past 40 years, breast cancer mortality has not decreased significantly while prostate cancer mortality has risen steadily; colon and rectum cancer mortality also remains high.[30] Chemotherapy is not winning the war against cancer. In contrast, during this same period, research in mice, and more recently in rats and humans, LDR was shown with high statistical confidence to be very effective in preventing and treating cancer. Human clinical trials have shown this immunotherapy to be much more effective in treating intermediate and high-grade stages of non-Hodgkin's lymphoma. Intensive further research during clinical trials is needed to optimize course protocols of LDR immunotherapy and, when indicated, the optimal interval between courses of LDR immunotherapy. LDR, in contrast to chemotherapy, stimulates rather than depresses all components of the antimutagenic biosystem and is asymptomatic without significant side effects. Published results of LDR immunotherapy justify current initiation of clinical trials in patients with breast, prostate and colorectal cancer.
VI. CONCLUSION
Recent research has led to recognition of the importance of the immune system in controlling cancer as well as infectious disease. LDR cancer immunotherapy has been shown to be effective in rodents and humans. Optimal protocols need to be developed by determining the mechanisms, magnitude and duration of immune response, and the optimal body localization of LDR needed to minimize marrow irradiation while maintaining maximal immune stimulation. Published results justify current support of well-designed clinical trials of LDR therapy in patients with breast, prostate, colorectal, ovarian cancer, and lymphomas. Clinical trials are also indicated to determine the effectiveness of LDR immune stimulation in patients with early HIV and other infectious diseases, and of LDR potentiation of vaccines to prevent HIV and other infectious diseases. LDR of patients is asymptomatic with minimal side effects, a rational and very promising way of using our antimutagenic system to control cancer and infection.
REFERENCES
- 1.Slavin S. Cancer immunotherapy with alloreactive lymphocytes. N Engl J Med. 2000;343:802–803. doi: 10.1056/NEJM200009143431109. [DOI] [PubMed] [Google Scholar]
- 2.Childs R, Chernoff A, Contentin N, et al. Regression of metastic renal cell carcinoma after non-myeloablative allogenic peripheral blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 3.Safwat A, Bayoumi Y, El-Sharkawy N, et al. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-Hodgkin's lymphoma. Radiother Oncol. 2003;69(1):33–36. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- 4.Safwat A. The role of low-dose total body irradiation in treatment of non-Hodgkin's lymphoma: a new look at an old method. Radiation and Oncology. 2000;56:1–8. doi: 10.1016/s0167-8140(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 5.Safwat A. The immunology of low-dose total body irradiation: more questions than answers. Radiat Res. 2000;151:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Richaud PM, Soubeyran P, Eghbali J, et al. Place of low-dose total body irradiation in the treatment of localized follicular non-Hodgkin's lymphoma: results of a pilot study. Int J Radiat Oncol Biol Phys. 1998;40(2):387–390. doi: 10.1016/s0360-3016(97)00722-0. [DOI] [PubMed] [Google Scholar]
- 7.Ueno RT, Rondon G, Mirza NQ, et al. Allogenic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol. 1998;16:986–993. doi: 10.1200/JCO.1998.16.3.986. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto K, Myogin M, Hosoi Y, et al. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9:161–175. [Google Scholar]
- 9.Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effects of inducible protective responses in mitigating endogenous damage. Human & Exp Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. [DOI] [PubMed] [Google Scholar]
- 10.Mille RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; UNSCEAR 1994 Report to the General Assembly, with Scientific Annexes. New York; Annex B. Adaptive Responses to Radiation in Cells and Organisms: 185–272
- 12.Feinendegen LE, Loken MK, Booz J, Muhlensiepen H, Sondhaus CA, Bond VP. Cellular mechanisms of protection and repair induced by radiation exposure and their consequences for cell system responses. Stem Cells. 1995;13(Suppl 1):7–20. [PubMed] [Google Scholar]
- 13.Feinendegen LE, Sondhaus CA, Bond VP, Muhlensiepen H. Radiation effects induced by low doses in complex tissue and their relation to cellular adaptive responses. Mutation Res. 1996;358:199–205. doi: 10.1016/s0027-5107(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamoka K. Increased SOD activities and decreased lipid peroxide in rat organs induced by low X-radiation. Free Radical Biol Med. 1991;11:3–7. doi: 10.1016/0891-5849(91)90127-o. [DOI] [PubMed] [Google Scholar]
- 15.Le XC, Xing JZ, Lee J, Leadon SA, Weinfeld M. Inducible repair of thymine glycol detected by an ultra sensitive assay for DNA damage. Science. 1998;280:1066–1069. doi: 10.1126/science.280.5366.1066. [DOI] [PubMed] [Google Scholar]
- 16.Ghiassi-nejad M, Mortazavi SMJ, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation area in Ramsar, Iran: preliminary biological studies. Health Phys. 2002;22:87–93. doi: 10.1097/00004032-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Makinodan T. Cellular and subcellular alteration in immune cells induced by chronic, intermittent exposure in vivo to very low dose of ionizing radiation (ldr) and its ameliorating effects on progression of autoimmune disease and mammary tumor growth. In: Sugahara T, Sagan LA, Aoyama T, editors. Low Dose Irradiation and Biological Defense Mechanisms. Amsterdam: Exerpta Medica; 1992. pp. 233–237. [Google Scholar]
- 18.Pollycove M, Feinendegen LE. Biologic responses to low doses of ionizing radiation: detriment versus hormesis. Part 2: Dose responses of organisms. J Nucl Med. 2001;42(9):26N–37N. [PubMed] [Google Scholar]
- 19.Pollycove M, Feinendegen LE. Molecular biology, epidemiology and the demise of the linear no-threshold (LNT) hypothesis. CR Acad Sci, Paris, Life Sciences. 1999;322:197–204. doi: 10.1016/s0764-4469(99)80044-4. [DOI] [PubMed] [Google Scholar]
- 20.Pollycove M. Nonlinearity of radiation health effects. Env Health Perspec. 1998;106:363–368. doi: 10.1289/ehp.98106s1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollycove M. Positive health effects of low level radiation in human populations. In: Calabrese EJ, editor. Biological Effects of Low Level Exposures: Dose-Response Relationships. Chelsea, MI: Lewis Publishers; 1994. pp. 171–187. [Google Scholar]
- 22.Anderson RE. In: Effects of low-dose radiation on the immune response. Chap 5 in: Biological Effects of Low Level Exposures: Dose-Response Relationships. Calabrese EJ, editor. Chelsea, MI: Lewis Publishers; 1992. pp. 95–112. [Google Scholar]
- 23.Makinodan T, James SJ. T cell potentiation by low dose ionizing radiation: possible mechanisms. Health Phys. 1990;59(1):29–34. doi: 10.1097/00004032-199007000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Miller AB, Howe GR, Sherman GJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examination in patients being treated for tuberculosis. N Engl J Med. 1989;321:1285–1289. doi: 10.1056/NEJM198911093211902. [DOI] [PubMed] [Google Scholar]
- 25.Cuttler JM, Pollycove M. Can cancer be treated with low doses of radiation? J Am Phy Surg. 2003;8(4):108–111. [Google Scholar]
- 26.Hashimoto S, Shirato H, Hosokawa M, et al. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- 27.Chaffey JT, Rosenthal DS, Moloney WD, Hellman S. Total body irradiation as treatment for lymphosarcoma. Int J Radiat Oncol Biol Phys. 1976;1:399–405. doi: 10.1016/0360-3016(76)90004-3. [DOI] [PubMed] [Google Scholar]
- 28.Choi NC, Timothy AR, Kaufman SD, Carey RW, Aisenberg AC. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin's lymphoma. Cancer. 1979;43:1636–1642. doi: 10.1002/1097-0142(197905)43:5<1636::aid-cncr2820430512>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Takai Y, Yamada S, Nemoto K, et al. Anti-tumor effect of low-dose total or half-body irradiation and changes in the functional subset of peripheral blood lymphocytes in non-Hodgkin's lymphoma patients after TBI (HBI) In: Sugahara T, Sagan LA, Aoyama T, editors. Low Dose Irradiation and Biological Defense Mechanisms. Amsterdam: Elsevier Science BV; 1992. pp. 113–116. [Google Scholar]
- 30.American Cancer Society Cancer statistics. CA. 2003;53(1):7–15. doi: 10.3322/canjclin.53.1.27. [DOI] [PubMed] [Google Scholar]