Abstract
Parthenium hysterophorus L. is an invasive weed that biosynthesizes several phytochemi-cals. The sesquiterpene lactone parthenin receives most attention regarding allelopathy of the plant or potential herbicidal properties. Since parthenin exhibits dose-dependent phy-totoxicity with low dose stimulation, this study investigated the occurrence and temporal features of parthenin hormesis in Sinapis arvensis L. sprayed with parthenin under semi-natural conditions. Dose/response studies showed that the occurrence and the magnitude of hormesis depended on climatic conditions and the parameter measured. Within the tested dose range, stimulatory responses were only observed under less-stressful conditions and were most pronounced for leaf area growth [138 % of control; 13 days after treatment (DAT)]. Temporal assessment of leaf area development showed that doses causing a stimulatory response at the end of the experiment (< 0.42 ± 0.04 kg/ha; 13 DAT) were initially inhibitory up to ED50 values (2 DAT). This clearly demonstrated an over-compensatory response. Inhibition of leaf area at 13 DAT reached ED50 values on average at 0.62 ±0.12 kg/ha, and S. arvensis was completely inhibited at doses exceeding 1.81 ±0.56 kg/ha (ED90). Based on these findings, implications of parthenin hormesis are discussed with respect to allelopathy of P. hysterophorus and exploitation of growth stimulatory responses in agriculture.
Keywords: dose/response modeling, dose/time responses, hormesis, Sinapis arvensis, spray application
INTRODUCTION
Biphasic dose/response relationships that are characterized by a stimulatory response in the measured parameter at low doses of a stressor and inhibition at higher doses are well recognized in toxicology and pharmacology (Calabrese and Baldwin 2001b; Calabrese and Blain 2005). This dose/response phenomenon is termed hormesis and represents an evo-lutionarily conserved process of adaptive, potentially beneficial responses to low doses of a stressor agent/condition (Calabrese et al. 2007). It has been observed with many chemically diverse compounds, with a wide range of measured endpoints, and in essentially all organisms studied so far. Still, little documentation exists on hormetic dose responses in plants, whether effects of synthetic herbicides or natural phytotoxins are concerned (Duke et al. 2006). Especially in the field of allelopathy, defined as both harmful and beneficial biochemical interactions among plants mediated by the release of plant-produced phytotoxins, reports of horme-sis are rare. Examples of hormesis with natural phytotoxins are reported with single compounds (e.g. gramine and hordenine, benzoxazolin-2(3H)-one, and allyl isothiocyanate), plant extracts (e.g. Triticum aestivum L.), and root exudates (e.g. T. aestivum and Triticum spelta L.) (An et al. 1993; Belz et al. 2005, 2007c). Based on these reports, it was hypothesized that hormesis may be a widespread dose/response feature of allelopathic phytotoxins, also referred to as allelochemicals (Rice 1984; An et al. 1993; Liu et al. 2003).
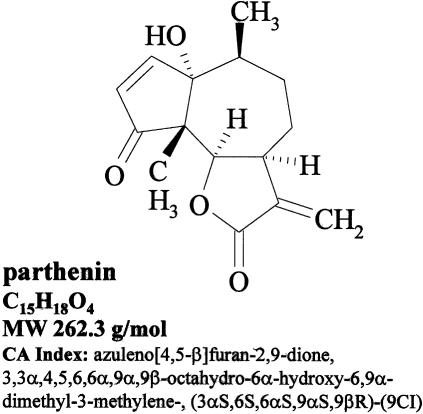
The sesquiterpene lactone parthenin produced by certain populations of the invasive weed Parthenium hysterophorus L. is another example for the stimulation/inhibition properties of some allelochemicals (Figure 1). The compound is biosynthesized during the entire life cycle of the plant, reaching maximum values during generative stages (Reinhardt et al. 2006). It is sequestered in capitate-sessile trichomes on leaves, stems, and the achene-complex of P. hysterophorus (Reinhardt et al. 2004). Parthenin is released from plant material by being washed from ruptured trichomes or from decomposing tissues and may contribute to the plant's interference with surrounding neighbors. Laboratory studies described parthenin's phytotoxic properties against a broad range of plant species, including weeds and crops [e.g. Ageratum conyzoides L., Amaranthus viridis L., Avena fatua L., Cassia tora L., Chenopodium murale L., Phaseolus aureus Roxb., and T. aestivum (Batish et al. 1997b, 2002a,b; Datta and Saxena 2001)]. The focus of these studies was adverse effects and, thus, recognition of parthenin hormesis was often constrained by the lack of doses below inhibition range.
FIGURE 1.
Structure of parthenin.
Therefore, stimulatory effects were at first only reported in association with plant extracts of P. hysterophorus (Table 1). Although parthenin was isolated as the major extract constituent, it is just one of several phy-tochemicals in this extract and, thus, a causal involvement in the observed extract hormesis remained initially unverified. Subsequent dose/response studies with the pure compound provided a first indication for the existence of hormesis by parthenin (Batish et al. 1997a,b), and complete dose/response modeling finally proved that parthenin significantly stimulates growth at low doses (Belz et al. 2007a). Pure parthenin treatments in equimolar amounts as present in extracts could reproduce the stimulatory response, which suggests that parthenin may play a key role in eliciting observed extract hormesis (Belz et al. 2007a).
TABLE 1.
Some examples of actual/presumed hormesis related to Parthenium hysterophorus.
| treatment | test plant | increased parameter | reference |
| parthenin | |||
| Echinochha crus-galli | root length* | ||
| Eragrostis curvuUi | root length* | Belz et al. 2007a | |
| Phaseolus aureus | shoot dry weight* | ||
| shoot length* | Batish et al. 1997a | ||
| Tnticum aestivum | root/shoot length | Batish et al. 1997b | |
| aqueous extracts of plant material | |||
| leaves (fresh material) | Eragrostis tef | root length* | Belz et al. 2007a |
| leaves (dry material) | Tnticum aestivum | shoot fresh weight | Pandey et al. 1993b |
| leaves, flower, stem, root (dry material) | Oryza sativa | carotenoid content | |
| chlorophyll content fresh weight | |||
| root/shoot length | Pandey, 1994b | ||
| leaves, flower, stem, root (dry material) | Eichiwrnia crassipes | fresh weight | |
| no. of healthy leaves | Pandey et al. 1993b | ||
| Salvinia molesta | fresh weight | ||
| no. of healthy fronds | Pandey 1994a,b |
statistical significance verified
Taking this into account, two aspects received consideration in the present study. From a practical point of view, stimulatory effects on crop plants suggest a potential use as a growth promoter. This application might come into focus with the renewed attention on herbicide-related hormesis (Cedergreen et al. 2005, 2007; Duke et al. 2006) and alternative uses of herbicides (Duke et al. 2007). As parthenin proved to be fairly unstable in a soil environment (Belz et al. 2007b), treatments of above-ground plant parts may be more promising in this regard and hormetic effects of parthenin on shoot elongation and dry weight of P. aureus point to the possibility (Batish et al. 1997a). Therefore, this study investigated the bioactivity of parthenin applied as a conventional spray application under semi-natural conditions. Experiments used Sinapis arvensis L. as a model test species and evaluated bioactivity of parthenin on different growth parameters as well as temporal features of dose responses.
A second aspect related to parthenin bioactivity is the question of the ecological significance and possible impacts of hormesis. Parthenin can be released by leaching from living plant parts or by decomposition of plant residues (Kanchan and Jayachandra 1980a; Reinhardt et al. 2004). While the latter mode of release is believed to release inhibitory levels of parthenin, it is still unknown if leaching of parthenin by rain, mist or dew is of a magnitude sufficient to cause a biological effect, whether stimulatory or inhibitory. Dropping of parthenin-containing leachates on leaves of target plants growing under the canopy of P. hysterophorus may be one mechanism of the plant's allelopathic capacity. Literature reports from other plant species demonstrated that such natural leachates can exhibit inhibitory allelopathic effects, e.g. Acacia dealbata Link (Carballeira and Reigosa 1999). However, phytotoxin concentrations found under field conditions in leachates are often below bioactive levels, e.g. Cucurbita spp. (Fujiyoshi et al. 2002). Thus, investigating the bioactivity in case of a spray application also establishes effective doses as a base to assess the significance of direct effects of parthenin dropped on the leaf surface of target plants by leaching from P. hysterophorus plant parts.
MATERIALS AND METHODS
Isolation of parthenin
The sesquiterpene lactone was isolated from organic solvent extracts of the leaf surface of greenhouse grown plants of a South African population of P. hysterophorus as described by Belz et al. (2007a). Extracts were obtained by dipping fresh leaf material for 10 s in tert-butyl methyl ether (250 mg/ml) followed by a filtration over anhydrous sodium sulphate. Vacuum concentrated extracts were redissolved in tert-butyl methyl ether and parthenin crystallized after heating (40 °C) by addition of small amounts of 2,2,4-trimethylpentane. After the supernatant was removed, parthenin crystals were washed twice with tert-butyl methyl ether and finally dried with nitrogen gas. Purity of isolated parthenin was checked using HPLC-DAD analysis (≥95 %) and results confirmed by HPLC-ESI−-MS.
Experimental design
Cultivation of plants
Seeds of S. arvensis (Herbiseed, Great Britain) were pregerminated in vermiculite for seven days and transplanted to 8 × 8 cm Jiffy-stripes (BayWa, Germany) filled with compost soil at a plant density of two plants/cavity. Plants were cultivated for another eight days under semi-field conditions in a vegetation hall at the University of Hohenheim, Stuttgart, Germany, prior to parthenin treatment. Plants were located in the glass roofed part of the vegetation hall that simulates outdoor conditions under rain protection. Ambient climatic conditions during the trial period (July/August 2006) are given in Table 2 (data represent outdoor conditions). Watering of plants was done with tap water as required.
TABLE 2.
Climate data of Stuttgart-Hohenheim (Ion. 9.21, lat. 48.7, 407 m NN).
| weather condition | July 2006 | August 2006 | |
| average air temperature (min./max.) | [°C] | 22.9 | 15.5 |
| (14.0/34.0) | (7.7/26.6) | ||
| average max. temp. | [°C] | 29.0 | 20.1 |
| average min. temp | [°C] | 17.1 | 12.0 |
| days > 25 °C | [no.] | 29 | 2 |
| days > 30 °C | [no.] | 13 | 0 |
| sunshine duration | [hrs] | 323.4 | 129.4 |
| sum of global radiation | [cm−2d−1] | 75628.0 | 44828.0 |
| average relative humidity | [%] | 64 | 79 |
Spray application
Fifteen days after germination, plants at the 2-leaf stage were treated with parthenin using a laboratory sprayer equipped with a flat-fan nozzle (type 8004, TeeJet-Spraying Sytems, USA; 2.5 bar pressure; 400 L/ha spray volume). Plants were treated with up to 11 doses of parthenin (0–3 kg/ha) prepared with deionized water (incl. 0.2 % Tween ®20, Roth) from stock solutions of parthenin in dimethyl sulfoxide. The final concentration of dimethyl sulfoxide at each treatment was 10 %. Controls were performed with solvent only and replicated 12 times. Parthenin treatments used one Jiffy-stripe each with six replications and were cultivated in a completely randomized design as described above.
Evaluation
The leaf area of plants was estimated non-destructively at 2, 4, 6, 8, 11, and 13 days after treatment (DAT) using a bi-spectral camera (IR spectrum > 680 nm; VIS spectrum 620–660 nm). As the distance between leaves and camera varied with time, concentration, and insertion on the stem, overarm images merely give an estimate of the real leaf area. Grey scale images were binarised using an image processing procedure with manually chosen thresholds and the relative coverage of leaves was determined from thresholded images (1024 × 766 pixel) as ratio of white to black pixels. Shoot length was recorded at 14 DAT as well as dry weight of aboveground plant parts after drying at 80 °C for three days.
Statistical analysis
The relationship between the average response (y) of dry weight, shoot length, or relative leaf area and parthenin concentration (x) was modeled by the modified four parameter logistic regression model of Cedergreen et al. (2005):
where c denotes the lower asymptote at infinite doses, d denotes the mean response of the untreated control, and f denotes the theoretical upper bound of the hormetic effect. The remaining parameters α, b, and e have no straightforward biological meaning, nevertheless, the size of b determines the steepness of the curve at inhibitory doses, e gives a lower bound on the ED50 (dose causing 50 % inhibition), and α is proportional to the rate of increase in responses at subinhibitory doses (Cedergreen et al. 2005). The model was fitted to data by nonlinear regression analysis using R (R Development Core Team 2006) with the add-on package drc (Ritz and Streibig 2005). Variance of responses was stabilized by an optimal box-cox transformation and the quality of curve fitting was assessed by F test for lack-of-fit based on an analysis of variance (P = 0.05). The value of α was fixed to 0.5 according to the smallest residual sum of squares resulting for the three built-in hormesis functions of the drc package (Cedergreen et al. 2005, 2007). Significance of hormesis was assessed by the 95 % confidence intervals (CI95) for the estimates of f and was given for f > 0. In such cases, where the 95 % confidence interval for f included zero, f was set to zero and equation 1 reduced to a monotonic decreasing four parameter logistic function. In case of hormesis, the dose M giving maximum response, the maximal response ymax, and the dose where the hormetic effect has disappeared or the limited dose for stimulation LDS [corresponds to ED1 and is also called ED/EC0 or no-observable-adverse-effect-level (NOAEL) (Cedergreen et al. 2007)] were computed using the drc package (Ritz and Streibig 2005). Comparison of dose/response curves was done by horizontal assessment (F test, P= 0.05) for similarity in upper and lower limits, b parameters and e values. Relative potencies of curves were assessed at the ED10 and ED50 levels.
RESULTS AND DISCUSSION
Bioactivity of parthenin
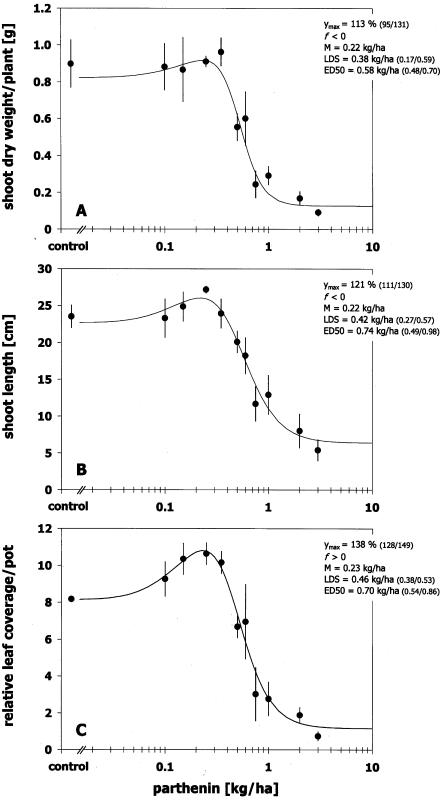
Dose responses of S. arvensis to leaf-applied parthenin showed a biphasic relationship (Figure 2). However, the magnitude and, thus, the significance of hormesis at the end of the experiment (13/14 DAT) varied depending on the parameter studied (Figure 3). Significance of hormetic effects could be statistically assessed only for the stimulation in relative leaf area [38 % maximum stimulation compared to control (CI95 28/49); f = 33.1 (CI95 2.2/63.9)]. f-Values for dose responses in shoot dry weight [f = 1.0 (CI95 −1.8/3.8)] and shoot length [f = 47.8 (CI95 −33.8/129.4)] were not significantly different from zero, although both endpoints showed a stimulatory trend [13 % stimulation in dry weight (CI95 −5/31); 21 % stimulation in shoot length (CI95 11/30)]. These results strengthen the importance of the response parameter for interpretation of hormesis, as hormetic dose responses are endpoint-specific. Owing to a supposed differential binding to stimulatory and/or inhibitory receptors or resource trade-offs for optimized growth under stressful conditions, dose responses in different traits do not necessarily correlate (Calabrese and Baldwin 2001a, 2002a; Cedergreen et al. 2007; Duke et al. 2006). For example, the herbicide metsulfuron-methyl stimulated leaf length of Hordeum vulgare L. at low doses, while total dry weight remained unaffected (Cedergreen et al. 2005). In this study, a stimulatory trend was observed for all endpoints measured and, thus, a trade-off between traits or a differential binding is not indicated. Batish et al. (1997a) correspondingly found stimulatory responses in seedling length and dry weight of P. aureus exposed to parthenin. Whether the stimulation of several plant traits by parthenin will result in an overall improvement of plant fitness over the long term is yet to be proven. Besides the endpoint measured, hormetic dose responses can vary depending on other factors of the study design, including doses used and species tested. For example, Batish et al. (2002b) also investigated the bioactivity of parthenin in a spray application, but did not observe hormetic effects, as doses used were inhibitory. The magnitude of hormesis on S. arvensis in this study was in the range of average hormesis responses observed for various herbicides in plants and algae (20–30 %; Cedergreen et al. 2005, 2007). However, maximum hormesis effects by parthenin on root length in germination assays reached higher values of up to 82 % stimulation using Eragrostis curvula (Schrad) Nees and Echinochloa crus-galli (L.) P. Beauv. as test species (Belz et al. 2007a).
FIGURE 2.
Dose responses of Sinapis arvensis on parthenin at 14 days after treatment (exp. 2, August 2006).
FIGURE 3.
Effect of parthenin on growth of Sinapis arvensis at 13/14 days after spray application (exp. 2, August 2006). (A) shoot dry weight, (B) shoot length, (C) relative leaf area. Bars indicate ± standard error of means. Ymax = maximal response; f = theoretical upper bound of the hormetic effect; M = dose giving maximum stimulation; LDS = limited dose for stimulation or ED1 (dose causing 1 % inhibition); 95 % confidence interval for estimates in parentheses.
Although the amplitude of parthenin hormesis varied with endpoint in this study, the stimulatory dose range corresponded. Maximum stimulation appeared on average at parthenin doses of 0.22 ± 0.01 kg/ha (M ± standard deviation) and disappeared at doses exceeding 0.42 ± 0.04 kg/ha (LDS). Thus, the mean distance between M and LDS doses comprised a 1.87 ± 0.12 fold increase in concentration, which is rather small compared to literature reports of an average 5 fold dose difference (Calabrese and Baldwin 2002a,b). An equally narrow stimulatory dose range for parthenin was observed in germination assays for root growth of E. curvu-la and E. crus-galli with an average 2.10 ± 0.02 fold increase in concentration (Belz et al. 2007a). In contrast to this, wider distances were observed in herbicidal studies [6 to 26 fold (Cedergreen et al. 2005, 2007)]. The limited quantitative features of parthenin hormesis observed in different biological models indicate that stimulation by parthenin may rather represent an overcompensation stimulation hormesis than a direct stimulation. In the latter case, a broader hormetic dose range (up to ≥103 fold) and a higher amplitude of the stimulatory response (up to 2 fold of control) would be expected (Calabrese 1999, 2001; Calabrese and Baldwin 2002b). Furthermore, a narrow stimulatory dose zone may indicate that only few mechanisms underlie the hormetic response (Calabrese and Baldwin, 2002b). Both hypotheses still lack sufficient biological understanding of parthenin hormesis to allow for a definite conclusion.
ED50 values ranged between 0.58 kg/ha (CI95 0.48/0.70) for dry weight to 0.70 kg/ha (CI95 0.54/0.86) for relative leaf area and 0.74 kg/ha (CI95 0.49/0.98) for shoot length. Thus, the dose range of apparent stimulation was at all endpoints at concentrations between zero and 65 ± 5 % of the ED50 which is somewhat higher than observed in herbici-dal studies (average upper limit of 20–25 %; Cedergreen et al. 2005, 2007), but equals observations in laboratory studies with parthenin (average upper limit of 68 %; Belz et al. 2007a). This may result from the narrow stimulatory dose range observed for parthenin in contrast to herbicidal studies and indicates that the span between stimulation and inhibition for parthenin is small. With average ED90 doses of 1.34 ± 0.33 kg/ha for all endpoints, parthenin proved to be a moderately effective herbicidal compound for the control of S. arvensis. This relatively weak activity, along with the observed hormesis effect, may account for the fact that parthenin has not been considered as a natural pesticide for weed management since its discovery in 1959 (Herz and Watanabe 1959). Furthermore, considering the limited solubility of parthenin in water and the observed effective doses, it seems unlikely that leaching of parthenin from intact leaves may be of a magnitude sufficient to have a direct lethal effect if dropped on plants growing under the canopy of P. hysterophorus.
Temporal features of parthenin bioactivity
Evaluating multiple time points, Pandey et al. (1993a) observed that low concentrated leaf extracts of P. hysterophorus initially reduced biomass development of the aquatic weed Eichornia crassipes (Mart) Solms, followed by a recovery of plants and increase in biomass compared to the untreated control. Pandey et al. (1993a) suggested a nutritional effect, but in consideration of apparent hormesis, it may have been an over-compensation response following initial injury by low levels of inhibitors in the extract. If parthenin plays in fact a decisive role for hormesis of leaf extracts of P. hysterophorus, bioactivity of the single compound should exhibit a comparable temporal feature.
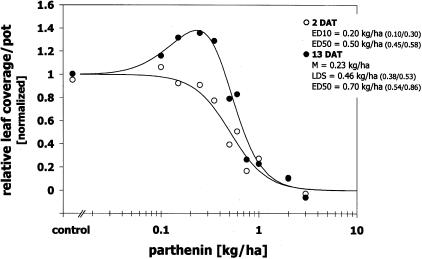
Present dose/time evaluations showed that S. arvensis plants were visually damaged from parthenin treatments starting from the first day after application. Visual symptoms mainly involved local necrosis on leaf surfaces, most likely caused by a direct contact effect. At 2 DAT this resulted in a strictly decreasing dose/response relationship with inhibitory effects on relative leaf area at doses exceeding 0.20 kg/ha (CI95 0.10/0.30; ED10) and a 50 % inhibition at 0.50 kg/ha (CI95 0.45/0.58) (Figure 4). A significant direct stimulatory effect on leaf area development was thus neither visible at the first day after treatment nor measurable at 2 DAT. Dose/response relationships at 4 and 6 DAT did not display a hormetic trend as well, in contrast to dose responses at 8, 11 and 13 DAT, although hormesis was significant only at 13 DAT. This demonstrates that plants recovered from the initial injury and overcompensated for inhibitory effects within a certain dose range. Comparing dose/response relationships at 2 and 13 DAT clearly showed that doses inducing stimulatory responses at 13 DAT (0.23–0.46 kg/ha and lower), decreased leaf area initially between 10 to 50 % (Figure 4). An initial 10 % inhibition caused a maximum stimulation at the end of the experiment and plants showing an initial 50 % inhibition recovered to response levels of the untreated control (LDS). Other test systems were able to overcompensate after an initial 100 % inhibition, which shows that over-compensatory responses can occur across a broad range of initial inhibitory dose levels (Calabrese 1999, 2001). This may suggest that the observed maximum of 50 % initial inhibition for overcompensatory responses might have been higher if the experiment had been extended. On the other hand, current findings correspond with overcompensated inhibition levels observed for the treatment of Mentha x piperita L. with the synthetic herbicide phosfon (2,4-dichlorobenzyl tributylphosphoni-um chloride), where an initial growth inhibition of more than 50 % could not be compensated until five weeks after treatment (Calabrese 1999).
FIGURE 4.
Effect of parthenin on leaf area of Sinapis arvensis at two (2 DAT) and 13 days after spray application (13 DAT; exp. 2, August 2006). Responses are normalized to controls. M = dose giving maximum stimulation; LDS = limited dose for stimulation or ED1 (dose causing 1 % inhibition); EDK = dose causing K % inhibition; 95 % confidence interval for estimates in parentheses.
Observed temporal features of parthenin hormesis along with the limited quantitative features confirm the hypothesis that hormesis by parthenin may represent the overcompensation stimulation type of hormesis. This supports the assumption of Calabrese (1999, 2001) that growth hormesis in general represents an overcompensation to previous injury and, thus, a disruption in homeostasis. The molecular and physiological mechanism underlying this phenomenon in case of parthenin are however unknown. Based on literature reports and their own findings Batish et al. (1997a) assumed that parthenin may act as plant growth regulator comparable to plant auxins. Synthetic auxins have been shown to elicit hormesis (Morré 2000; Allender et al. 1997). Furthermore, Batish et al. (1997a, 2002b) assumed that parthenin may inactivate enzymes containing a thiol group by non-reversible alkylation. The same mechanism is supposed to underlie phytotoxicity of natural isothiocyanates (Drobnica et al. 1977) and for one compound of this group of phyto-chemicals, allyl isothiocyanate, marked hormesis was observed as well (Belz et al. 2007c). Thus, hormetic responses were observed in association with both hypothetical modes of action, but especially a potential growth regulatory action may open to exploit parthenin as a stimulant for crop seedling growth. This type of application was also proposed by Pandey (1994b) for plant extracts of P. hysterophorus. An essential prerequisite for a successful application as growth promoter is however a high repro-ducibility of hormetic responses.
Reproducibility of parthenin bioactivity
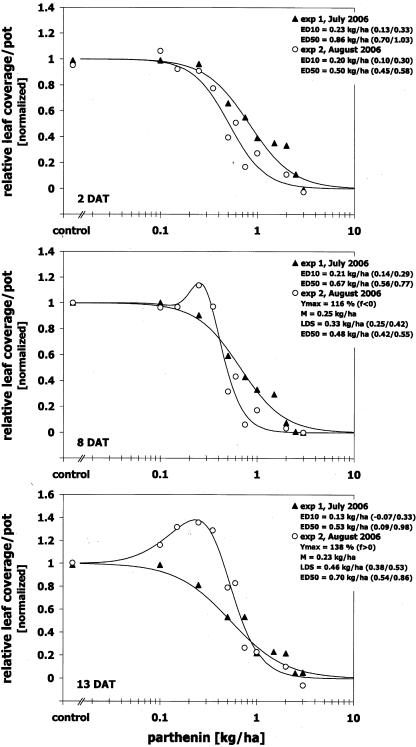
In order to evaluate reproducibility of dose responses by parthenin, experiments were carried out twice during July and August 2006 applying the same experimental protocol. Results showed that at 2 DAT, the two dose/response curves for relative leaf area were parallel, but shifted towards lower doses in the second experiment conducted in August (exp. 2). In comparison with the first evaluation in July (exp. 1), relative potencies were not significantly different at the ED10 dose level (0.22 ± 0.02 kg/ha), while the observed 1.7 fold shift to lower ED50 doses at exp. 2 was significant. Thus, the initial efficacy of parthenin was higher in exp. 2. Nevertheless, in both experiments the inhibitory dose range started at doses exceeding 0.22 ± 0.02 kg/ha, and dose/response curves were strictly decreasing (Figure 5; 2 DAT). The equivalent was observed for evaluations at 4 and 6 DAT, which indicated a roughly similar initial inhibition in both experiments. Despite this, dose/response curves developed to the contrary starting from 8 DAT. In exp. 2 plants began to overcompensate the initial inhibition at low doses, while no compensation occurred in exp. 1 (Figure 5; 8 DAT). By the end of the experiments dose responses were biphasic in exp. 2 and strictly decreasing in exp. 1 (Figure 5; 13 DAT). The dose M giving maximum stimulation in exp. 2 equaled the ED20 in exp. 1. Furthermore, ED50 values in exp. 2 increased from 0.50 kg/ha (CI95 0.45/0.58; 2 DAT) to 0.70 kg/ha (CI95 0.54/0.86; 13 DAT) and decreased in exp. 1 from 0.86 kg/ha (CI95 0.70/1.03) to 0.53 kg/ha (CI95 0.09/0.98). This shows that plants were not able to compensate inhibitory effects in exp. 1 and the efficacy of parthenin increased over time in contrast to plants in exp. 2.
FIGURE 5.
Effect of a spray application of parthenin on leaf area of Sinapis arvensis cultivated for two (2 DAT), eight (8 DAT), and 13 days after treatment (13 DAT) under semi-field conditions in July 2006 (exp. 1) and August 2006 (exp. 2) at Stuttgart-Hohenheim, Germany. Responses are normalized to controls. Ymax = maximal response; f = theoretical upper bound of the hormetic effect; M = dose giving maximum stimulation; LDS = limited dose for stimulation or ED1 (dose causing 1 % inhibition); EDK = dose causing K % inhibition; 95 % confidence interval for estimates in parentheses.
As the experimental protocol was equal in both experiments, it may be speculated that climatic conditions during the test period accounted for the observed disparity of results. With 29 days over 25 °C in exp. 1, it was much warmer than in exp. 2 where temperatures over 25 °C were only reached during two days (Table 2). Conditions in exp. 1 were further on characterized by a 2.5 fold longer photoperiod and a 1.7 fold higher sum of global radiation. Higher temperatures along with a more intense radiation in exp. 1 may have been responsible for the higher efficacy of parthenin at 13 DAT, a phenomenon that is especially known for temperature from studies with herbicides (e.g. Madafiglio et al. 2000; Fauser and Renner 2001). The higher efficacy may have shifted the stimulatory dose zone towards lower doses. Such a shift has been observed previously owing to experimental conditions (Calabrese and Baldwin 2002a,b), but it would not have been captured by the dose range tested.
Another hypothesis related to observed disparity of results is based on the fact that overcompensation hormesis requires resource allocation for adaptive responses. However, plants allocate their resources to maintain homeostasis (Calabrese and Baldwin 2002a,b; Cedergreen et al. 2007) and, thus, under stressful conditions as in exp. 1, resources for vegetative growth may have been limited due to trade-offs within the plant for other physiological stress responses. This hypothesis would be strengthened by the fact that absolute values of measured parameters were lower in exp. 1, e.g. controls in exp. 1 achieved merely 72 % of the relative leaf area of controls in exp. 2. However, studies investigating the influence of environmental conditions on the expression and temporal features of horme-sis in plants are yet absent.
Failure to reproduce bioactivity features under contrasting experimental conditions shows the importance of controlled conditions to reproduce hormesis results and the need to investigate the impact of environmental conditions on the dose/time expression of hormesis in plants.
Practical and ecological implications of parthenin bioactivity
Practical aspects. Although bioactivity of parthenin considerably varied depending on growing conditions, this study demonstrates the potential of exploiting hormetic effects for desired agronomic effects if conventionally applied. Applications of hormetic compounds may produce beneficial effects on quantitative and qualitative plant traits, e.g. glyphosate-mediated increase in sucrose content in sugarcane (McDonald et al. 2001), or plant fitness, e.g. elicitation of defenses against pathogens (Nelson et al. 2002). Although several of these applications have been proposed in the past, beneficial hormetic effects have not been used to any large extent (Duke et al. 2006). The reasons for this may be complex, but include variability of hormetic responses. At high doses, plants are mostly killed, leaving no great variability in responses. At stimulatory doses, the system is more variable, as here resources are allocated as needed to reestablish or maintain homeostasis (Calabrese and Baldwin 2002a,b). Furthermore, a desirable hormetic change in a certain plant trait may not necessarily conserve homeostasis under all growing conditions and, thus, may not always serve as a sink for resource allocation. Unpredictable long-term effects may place further constraints, as over time a hormetic change in one trait can be at the expense of another or at the expense of its own (Duke et al. 2006).
Ecological aspects. Considering ecological implications, this study may allow some insight into the significance of leaching of parthenin from intact leaves of P. hysterophorus by rain, mist or dew. One mode of biochemical interaction that is relevant in this context is dropping of leachates on leaves of target plants growing under the canopy of P. hysterophorus.
Despite the fact that parthenin could be identified in leaf washings (Kanchan and Jayachandra 1980a), reports on parthenin concentrations in leaf leachates that would allow for a comparison with observed effective doses are lacking. Any conclusion on the ecological impacts of parthenin leaching is thus speculative. Nevertheless, if the effective doses observed at the ends of both experiments are taken into account, an average dropping of more than 0.31 ± 0.26 kg/ha (ED10) would be necessary to inhibit the leaf area growth of S. arvensis and 1.81 ± 0.56 kg/ha (ED90) would be necessary to cause lethal effects. Based on literature reports, leaf mass of a dense stand of P. hysterophorus may theoretically bound 7–17 g parthenin/m2 (Rodriguez et al. 1976, Kanchan and Jayachandra 1980b, Reinhardt et al. 2006, Belz et al. 2007a). Thus, if more than 0.1–0.7 % of the parthenin stored in leaves at dense stands of P. hysterophorus may be released at once, adverse effects (> ED10) on sensitive plant species by dropping might be possible. Lethal effects (> ED90) would require up to 3.2 %. Aqueous extraction to simulate the natural release of parthenin during decay, removed approximately 10 % of the total amount present in the leaves within 24 h (Reinhardt et al. 2004). A release of up to one third of this amount by rain, mist or dew seems remote and, thus, a direct lethal effect by leaching may be doubtful. Whether up to one tenth can be removed and, thus, an inhibitory amount needs to be evaluated. Lower rates of release or sparse stands of P. hysterophorus may in contrast lead to stimulatory effects.
Several studies proved parthenin to elicit hormesis and, thus, it is possible that hormetic effects may occur in a natural setting if doses released are low. Moreover, the fate of allelochemicals in the environment may lead to stimulatory dose levels even if inhibitory doses are initially released (An et al. 2002, 2003). Therefore, hormesis should be regarded as a potential low dose component of plant/plant interference and in particular in case of parthenin-mediated interactions by P. hysterophorus.
CONCLUSIONS
The example of parthenin demonstrated how significant hormesis can be for bioactivity features of phytotoxins. Furthermore, the observed temporal features and sensitivity of parthenin hormesis to climatic conditions showed that this phenomenon is not just ‘trivial’ low dose stimulation. Despite a supposed high relevance of hormesis for natural phyto-toxins and allelopathic interactions, stimulatory effects of phytotoxins at low doses are rarely considered. Lack of consideration of hormesis hampers the assessment of its ecological significance and consequences in a natural environment as well as its exploitation for agricultural use. Considerable research will be needed in order to understand the ecological conditions necessary for hormesis and potential impacts, benefits, or risks of low dose stimulation by phytotoxins. The findings should be beneficial to allelopathy and agriculture.
ACKNOWLEDGMENTS
The author is grateful for the technical assistance provided by Christine Metzger, Peter Risser, and Martin Weis, as well as the constructive comments on the manuscript provided by three unknown reviewers and Dr. Stepnen O. Duke.
REFERENCES
- Allender WJ, Cresswell GC, Kaldor J, Kennedy IR. Effect of lithium and lanthium on herbicide induced hormesis in hydroponically-grown cotton and corn. J Plant Nutr. 1997;20:81–95. [Google Scholar]
- An M, Johnson IR, Lovett JV. Mathematical modeling of allelopathy: biological response to alle-lochemicals and its interpretations. J Chem Ecol. 1993;19:2379–2388. doi: 10.1007/BF00979671. [DOI] [PubMed] [Google Scholar]
- An M, Johnson IR, Lovett JV. Mathematical modeling of allelopathy: the effects of intrinsic and extrinsic factors. Plant Soil. 2002;246:11–22. [Google Scholar]
- An M, Liu DL, Johnson IR, Lovett JV. Mathematical modeling of allelopathy: II. The dynamics of allelochemicals from living plants in the environment. Ecol Model. 2003;161:53–66. [Google Scholar]
- Batish DR, Kohli KH, Saxena DB, Singh HP. Growth regulatory response of Parthenin and its derivatives. Plant Growth Regul. 1997a;21:189–194. [Google Scholar]
- Batish DR, Kohli KH, Singh HP, Saxena DB. Studies on herbicidal activity of parthenin, a constituent of Parthenium hysterophorus, towards billgoat weed (Ageratum conyzoides) Curr Sci. 1997b;73:369–371. [Google Scholar]
- Batish DR, Singh HP, Kohli RK, Saxena DB, Kaur S. Allelopathic effects of parthenin against two weedy species, Avena fatua and Bidens pilosa. Environ Exp Bot. 2002a;47:149–155. [Google Scholar]
- Batish DR, Singh HP, Saxena DB, Kohli RK. Weed suppressing ability of parthenin – a sesquiterpene lactone from Parthenium hysterophorus. NZ Plant Prot. 2002b;55:218–221. doi: 10.1023/a:1021089013754. [DOI] [PubMed] [Google Scholar]
- Belz RG, Duke SO, Hurle K. Dose-response - a challenge for allelopathy? Nonlinearity Biol Toxicol Med. 2005;3:173–211. doi: 10.2201/nonlin.003.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz RG, Reinhardt CF, Foxcroft LC, Hurle K. Residue allelopathy in Parthenium hysterophorus L. - does parthenin play a leading role? Crop Prot. 2007a;26:237–245. [Google Scholar]
- Belz, RG, Van der Laan M, Reinhadt CF, Hurle K. 2007b. Soil degradation of parthenin - does it contradict a role in allelopathy of the invasive weed Parthenium hysterophorus L.? Proceedings 14th EWRS Symposium, Hamar, Norway, 17–21 June 2007, p. 166.
- Belz RG, Velini ED, Duke SO. Dose/response relationships in allelopathy research. In: Fujii Y, Hiradate S, editors. Allelopathy. New concepts and methodology. New Hampshire, USA: Science Publishers; 2007c. pp. 3–29. [Google Scholar]
- Calabrese EJ. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotox Environ Safety. 1999;42:135–137. doi: 10.1006/eesa.1998.1729. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Overcompensation stimulation: a mechanism for hormetic effects. Crit Rev Toxicol. 2001;31:425–470. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Agonist concentration gradients as a generalizable regulatory implementation strategy. Crit Rev Toxicol. 2001a;31:471–473. doi: 10.1080/20014091111758. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001b;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Applications of hormesis in toxicology, risk assessment and chemotherapeutics. Trends Pharmacol Sci. 2002a;23:331–337. doi: 10.1016/s0165-6147(02)02034-5. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002b;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Carballeira A, Reigosa MJ. Effects of natural leachates of Acacia dealbata Link in Galicia (NW Spain) Bot Bull Acad Sin. 1999;40:87–92. [Google Scholar]
- Cedergreen N, Ritz C, Streibig JC. Improved empirical models describing hormesis. Environ Tox Chem. 2005;24:3166–3172. doi: 10.1897/05-014r.1. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Streibig JC, Kudsk P, Mathiassen SK, Duke SO. The occurrence of hormesis in plants and algae. Dose Responce. 2007;5:150–162. doi: 10.2203/dose-response.06-008.Cedergreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Saxena DB. Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Manage Sci. 2001;57:95–101. doi: 10.1002/1526-4998(200101)57:1<95::AID-PS248>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Drobnica L, Kristian K, Augustin J. 1977. The chemistry of the -NCS group. In: Patai S. (ed), The chemistry of cyanates and their thio derivates, part 2, pp. 1003–1221, John Wiley & Sons, NY, USA.
- Duke SO, Cedergreen N, Velini ED, Belz RG. Hormesis: is it an important factor in herbicide use and allelopathy? Outlooks Pest Manag. 2006;17:29–33. [Google Scholar]
- Duke SO, Wedge CE, Cerdeira AL, Matallo MB. Herbicide effects on plant disease. Outlooks Pest Manag. 2007;18:36–40. [Google Scholar]
- Fauser JC, Renner KA. Environmental effects on CGA-248757 and flumiclorac efficacy/soybean tolerance. Weed Sci. 2001;49:668–674. [Google Scholar]
- Fujiyoshi PT, Gliessman SR, Langenheim JH. Inhibitory potential of compounds released from squash (Cucurbita spp.) under natural conditions. Allelopathy J. 2002;9:1–8. [Google Scholar]
- Herz W, Watanabe K. Parthenin, a new guauanolide. J Am Chem Soc. 1959;81:6088–6089. [Google Scholar]
- Kanchan SD, Jayachandra Allelopathic effects of Parthenium hysterophorus L. Part IV. Identification of inhibitors. Plant Soil. 1980a;55:67–75. [Google Scholar]
- Kanchan SD, Jayachandra Allelopathic effects of Parthenium hysterophorus L. Part II. Leaching of inhibitors from aerial vegetative parts. Plant Soil. 1980b;55:61–66. [Google Scholar]
- Liu DL, An M, Johnson IR, Lovett JV. Mathematical modelling of allelopathy: III. A model for curve-fitting allelochemical dose-responses. Nonlinearity Biol Toxicol Med. 2003;1:37–50. doi: 10.1080/15401420390844456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madafiglio GP, Medd RW, Cornish PS, Van de Ven R. Temperature-mediated responses of flumetsulam and metosulam on Raphanus raphanistrum. Weed Res. 2000;40:387–395. [Google Scholar]
- McDonald L, Morgan T, Jackson P. The effect of ripeners on the CCS or 47 sugarcane varieties in the burdekin. Proc. Conf. Austral. Soc. Sugar Cane Technologists. 2001;23:102–108. [Google Scholar]
- Morré DJ. Chemical hormesis in cell growth: a molecular target at the cell surface. J Appl Toxicol. 2000;20:157–163. [PubMed] [Google Scholar]
- Nelson A, Renner KA, Hammerschmidt R. Effects of protoporhyrinogen oxidase inhibitors on soybean (Glycine max L.) response, Sclerotinia sclerotiorum disease development, and phytoalexin production by soybean. Weed Technol. 2002;16:353–359. [Google Scholar]
- Pandey DK, Kauraw LP, Bhan VM. Inhibitory effect of parthenium (Parthenium hysterophorus L.) residue on growth of waterhyacinth (Eichornia crassipes Mart Solms.). I. Effect of leaf residue. J Chem Ecol. 1993a;19:2651–2662. doi: 10.1007/BF00980698. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Kauraw LP, Bhan VM. Inhibitory effect of parthenium (Parthenium hysterophorus L.) residue on growth of waterhyacinth (Eichornia crassipes Mart Solms.). II. Relative effect of flower, leaf, stem, and root residue. J Chem Ecol. 1993b;19:2663–2671. doi: 10.1007/BF00980699. [DOI] [PubMed] [Google Scholar]
- Pandey DK. Inhibition of Salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hys-terophorus L). I. Effect of leaf residue and allelochemicals. J Chem Ecol. 1994a;20:3111–3122. doi: 10.1007/BF02033714. [DOI] [PubMed] [Google Scholar]
- Pandey DK. Inhibition of Salvinia (Salvinia molesta Mitchell) by parthenium (Parthenium hys-terophorus L.). II. Relative effect of flower, leaf, stem, and root residue on salvinia and paddy. J Chem Ecol. 1994b;20:3123–3131. doi: 10.1007/BF02033715. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2006. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
- Reinhardt C, Kraus S, Walker F, Foxcroft L, Robbertse P, Hurle K. 2004. The allelochemical parthenin is sequestered at high level in capitate-sessile trichomes on the leaf surface of Parthenium hysterophours. J Plant Dis Prot Special Issue XIX, 253–261.
- Reinhardt C, Van der Laan M, Belz RG, Hurle K, Foxcroft L. Production dynamics of the alle-lochemical parthenin in leaves of Parthenium hysterophorus L. J Plant Dis Prot Special Issue. 2006;XX:427–433. [Google Scholar]
- Rice EL. Allelopathy. 2nd ed. New York: Academic Press; 1984. [Google Scholar]
- Ritz C, Streibig JC. J Statist Software. Vol. 12. 2005. Bioassay analysis using R; pp. 1–22. [Google Scholar]
- Rodriguez E, Dillon MO, Mabry TJ, Mitchell JC, Towers GHN. Dermatologically active sesquiterpene lactones in trichomes of Parthenium hysterophorus L. (Compositae) Experientia. 1976;32:326–238. doi: 10.1007/BF01937785. [DOI] [PubMed] [Google Scholar]