Abstract
Confounding factors in radiation pulmonary carcinogenesis are passive and active cigarette smoke exposures and radiation hormesis. Significantly increased lung cancer risk from ionizing radiation at lung doses < 1 Gy is not observed in never smokers exposed to ionizing radiations. Residential radon is not a cause of lung cancer in never smokers and may protect against lung cancer in smokers. The risk of lung cancer found in many epi-demiological studies was less than the expected risk (hormetic effect) for nuclear weapons and power plant workers, shipyard workers, fluoroscopy patients, and inhabitants of high-dose background radiation. The protective effect was noted for low- and mixed high- and low-linear energy transfer (LET) radiations in both genders. Many studies showed a protection factor (PROFAC) > 0.40 (40% avoided) against the occurrence of lung cancer. The ubiquitous nature of the radiation hormesis response in cellular, animal, and epidemio-logical studies negates the healthy worker effect as an explanation for radiation hormesis. Low-dose radiation may stimulate DNA repair/apoptosis and immunity to suppress and eliminate cigarette-smoke-induced transformed cells in the lung, reducing lung cancer occurrence in smokers.
Keywords: Hormesis, Smoking, Lung Cancer
SMOKING AND CANCER
Cigarette smoke is a complex mixture of more than 6000 chemicals that are separated into a vapor phase and a particulate phase, and numerous carcinogenic compounds have been identified in primary and side-stream tobacco smoke. Hundreds of chemicals in tobacco smoke, of which more than 40 are known to cause cancer in humans and/or animals (Sanders 1996), react covalently with DNA to form adducts or produce free radicals causing oxidative damage.
Approximately one-third of all cancers in the United States are related to tobacco use, including active and passive cigarette smoke exposures and use of smokeless products (USDHHS 1990). The Surgeon General's annual report on smoking and health for 2004 listed causal relationships between smoking and cancer in the bladder; cervix; esophagus; kidney; larynx; lung, oral cavity; pharynx; pancreas and stomach; and for acute myeloid leukemia, and Hodgkin's lymphoma (USDHHS 2004). Lung cancer frequency in the United States is significantly influenced by race, gender, and lifestyle (ALA 2005), and approximately 20% of cigarette smokers will develop lung cancer. Lung cancer survival rates are poor, with only 10% of patients living longer than 1 yr after diagnosis, while smoking is responsible for over 90% of lung cancer deaths (Sethi 1997). The lung cancer incidence in Dresden has increased approximately 100fold from 0.06% in 1852 until today and is directly linked to cigarette production that began in Dresden in 1862 (Deetjen and Falkenbach 1999). For ex-smokers, the risk of lung cancer is highly dependent upon the number of cigarettes smoked per day, as well as the duration, and length of time since stopping smoking (USDHHS 1982). The relative risk (RR) for lung cancer in heavy smokers living in the United States, France, Spain, and Germany ranges from 20–46. The RR for lung cancer in France was 15.6 for current smokers, 9.2 for ex-smokers who stopped within the previous 10 yr, and 2.9 for ex-smokers who stopped at least 10 yr earlier; similar incidences of lung cancer are also seen in other Western countries (Sanders 1996; Rossi and Zaider 1997; Kreienbrock et al. 2001; Barros-Dios et al. 2002; Baysson et al. 2004; Wichmann et al. 2005).
INTERACTION OF RADIATION AND SMOKING
Over 100 separate studies of radium dial painters; hard rock and uranium miners; radiologists and radiological technicians; nuclear workers; X-ray technicians; residents with high indoor radon; radium therapy, thorotrast and ankylosing spondylitis patients; and Hodgkin disease, pedi-atric cancer; thymus hypertrophy, tinea capitis, metropathia hemorrhagi-ca, postpartum mastitis, fluoroscopy, peptic ulcer, female breast cancer, and benign breast disease patients have all failed to demonstrate a significant excess of lung cancer incidents at a lung dose < 1 Gy in never smokers (Sanders 1996). A meta-analysis of lung cancer risk after exposure to low-linear energy transfer (LET) radiations indicated that doses < 2 Gy did not appear to cause lung cancer, “but, in fact, indicate a reduction of the natural incidence” (Rossi and Zaider 1997).
Among never smokers, no increase in lung cancer has been found in flight attendants exposed during flight to elevated background radiation (Blettner et al. 2002); workers in a uranium processing plant (Dupree et al. 1995); inhabitants of the United States, India, China, or Iran who experience background radiation levels that are up to 200-fold higher than the mean global level (Zahi et al. 1982; Nambi and Soman 1987; NCRP 2001; Ankathil et al. 2005; Ghiassi-Nejad and Mortazavi 2005; Mortazavi et al. 2006); Japanese A-bomb survivors (Aurengo et al. 2005); radiologists and radiological technicians (Doody et al. 1998; Berrington et al. 2001; Yoshinaga et al. 2003); residents in the Eastern Urals exposed to radiation from the 1957 buried nuclear waste tank explosion near the Mayak plutonium production facility (Kostyuchenko and Krestina 1994); or Mayak facility workers receiving alpha radiation doses < 1 Gy in combination with gamma rays (Tokarskaya et al. 1997). Additionally, Cardis et al. (1995) found that lung cancer was not increased in nuclear industry workers from the United States, United Kingdom, and Canada. Evacuees and others living close to the exclusion zone around Chernobyl received whole-body doses in the range of 0.1 to 0.5 Gy, and average lung doses were as high as 0.6 Gy due largely to inhalation of radionuclides (Baverstock and Williams 2002). Furthermore, no evidence of an increase in the incidence of lung cancer was found in any group associated with the Chernobyl accident (Chernobyl Forum 2005; Hatch et al. 2005). The lowest dose for which lung cancer was found in non-smoking U.S. uranium miners was > 100 working-level months (WLM)† (Hornung and Meinhardt 1987).
Smoking accounts for a large share of deaths attributed to radiation — primarily lung cancers –– which are much higher in frequency than for other solid cancers. A heavy smoker may accumulate an alpha-radiation dose as high as 1 Gy, mostly from 210Po, to bronchial bifurcations (Martell 1975). The interaction of radiation and smoking exhibits a sub-multiplicative or multiplicative relationship in the induction of lung cancer (Martell 1975; Gray et al. 1986; Janerich et al. 1990; Monchaux et al. 1994; Thomas et al. 1994; Barros-Dios et al. 2002; Gilbert et al. 2003; Pierce et al. 2003; Wang et al. 2005). Cigarette smoking so confounds studies of lung cancer formation in irradiated populations that it is difficult to determine if ionizing radiation even exerts a significant effect on lung cancer formation in smokers with a RR < 1.5–2.0 (Doll 1992; Schull and Weiss 1992), and little is known about the interaction of smoking and radiation on non-respiratory tract cancers.
Eighty percent of male A-bomb survivors reported that they were smokers (Pierce et al. 2003); and of approximately 600 lung cancer cases in Japanese A-bomb survivors, approximately 50 were related to radiation using the linear no-threshold (LNT) hypothesis (Kopecky et al. 1986; Pierce et al. 1996). An increase in lung cancer in never smokers was not detected. These few “excess” lung tumors could be mostly explained by the uncertainty of smoking habits (Little 2002). Providing a risk factor based upon so few tumors and such uncertainty of smoking habits does not give much confidence as to their accuracy. Presently, there are no current methodologies for low tumor prevalence that can accurately discriminate between the different possible causes of cancer (Heidenreich et al. 2002).
Insufficient data was given in many published papers for others to evaluate lung cancer risk at low doses. Low-dose and smoking data used to determine the excess relative risk (ERR) for lung cancer in Mayak workers was not provided (Kreisheimer et al. 2000; Shilnikova et al. 2003; Gilbert et al. 2004). Lung cancer mortality estimates for the Semipalatinsk cohort, downwind from the Kazakhstan weapons test site, did not adequately control for smoking; no data was given for the 20–70 mSv cohort and data for the 70–249 mSv cohort was combined from several dose groups (Bauer et al. 2005). A previous Portsmouth Naval Shipyard worker-positive ERR value for lung cancer was negated by a later study that adjusted for smoking, welding fumes, and asbestos exposure (Yiin et al. 2005). Likewise, no data was given for exposures to asbestos or for smoking history in a study of lung cancer in Hanford workers (Wing and Richardson 2005). Cohorts of nuclear workers in 15 countries were evaluated in a meta-analysis of cancer risk estimation, but risk by dose level was not given for each study. The authors admit that their data may be confounded by smoking (Cardis et al. 2005).
RADIATION HORMESIS
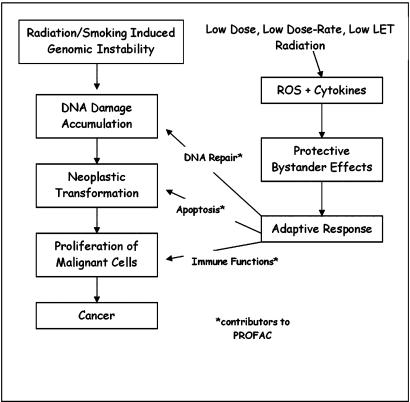
Hormesis is derived from the Greek word, hormaein, to excite. A current hormesis hypothesis states that low-level stress (e.g., via irradiation) stimulates a system of protective biological processes at the cellular, molecular, and organismic levels, decreasing cancer incidences and the incidences of other deleterious health effects below spontaneous levels (Scott 2006a,b,c) (Figure 1). Broadly based data showing hormesis has been found independently of chemical or physical agent, or biological model and endpoint (Calabrese 2005; Calabrese and Baldwin 2003).
FIGURE 1.
Possible radio-adaptive mechanisms that contribute to PROFAC during cigarette smoking-induced pulmonary carcinogenesis.
Recent research has led to the formulation of a sound scientific basis for radiation hormesis (Luckey 1991; Stephens et al. 1991; Calabrese and Baldwin 2000, 2001; Cohen 2002b; Scott 2005, 2006a,b; Scott et al. 2006). A biological basis for radiation hormesis has been proposed and relates to activated DNA repair, apoptosis (self destruction), and stimulation of the immune system (Scott et al. 2006). Cells carry in their genome a program for apoptosis, which limits the accumulation of potentially harmful cells. Failure to regulate tissue homeostasis can result in cancer development (Thompson et al. 1992). Cell regulators, such as growth factors, cytokines, and hormones, are involved with the activation and repression of genes associated with apoptosis. Stephens et al. (1991) found that apoptosis is enhanced by low to moderate doses of ionizing radiation in normal tissues and tumors; and Scott et al. (2006) notes that high fidelity DNA repair is also activated above a threshold dose.
Two forms of apoptosis (normal which is presumed to involve p53, along with an auxiliary protective apoptosis-mediated [PAM] process that is considered to be independent of p53) have been incorporated into a biologically based model of low-dose-induced stochastic radiobiological effects (Scott 2005, 2006a,b; Scott et al. 2006). Normal apoptosis backs up the DNA repair pathway by removing severely damaged cells to undergo repair. The auxiliary PAM process removes mutant, neoplastically transformed, and other genomically unstable cells that arise as a result of mis-repair (or no repair) of DNA damage (Scott 2005).
The PAM process, along with stimulation of the immune system, limits potential cancer formation when activated by low LET-radiations such as gamma rays and x-rays (Scott 2005, 2006a,b). Stimulation of the PAM process by low-dose, low-LET radiation may cause apoptosis of cells transformed by the other agents, including chemical carcinogens in cigarette smoke. A significant suppression of 20-methylcholanthrene carcinogene-sis was found in ICR female mice given gamma-irradiation at 1 mGy/h (Sakai et al. 2003). Mice exposed dermally to 50 cGy beta-irradiation 24 h prior to treatment with the chemical carcinogen methyl-nitro-nitroso guanidine reduced papilloma frequency by approximately 5-fold (Mitchel et al. 1999). Low-dose ionizing radiation may enhance the elimination, by apoptosis, of cigarette-induced transformed pulmonary cells, thus decreasing lung cancer risk (Scott 2006b). Activated high fidelity DNA repair by low doses of low-LET radiation is also considered a contributing factor in suppressing the occurrence of stochastic effects of other carcinogens.
In his book, Radiation Hormesis, Luckey (1991) describes evidences of hormesis in workers at nuclear facilities, A-bomb survivors, and many other groups exposed to low doses of radiation. The hormesis response is associated with increased lifespan as well as decreased mutations, chromosome aberrations, neoplastic transformations, congenital malformations, and cancer (Lorenz et al. 1955; Brown et al. 1963; Frigerio et al. 1973; Jaworowski 1995; Spalding et al. 1982; Wei 1997; Caratero et al. 1998; Kant et al. 2003).
Relative Risk and the Protection Factor
A novel biological-based hormetic relative risk (HRR) model for cancer induction by low-dose, low-dose-rate irradiation has emerged out of concern about the inappropriateness of the LNT hypothesis (Scott 2006a,b,c). A low-dose form of the HRR model is presented below and is used to characterize, in a systematic manner, hormetic responses observed in many irradiated human populations.
Based on the demonstrated close similarity between the RR dose-response relationships for radiation-induced neoplastic transformation in vitro and for cancer induction in humans (Redpath et al. 2001), a novel, RR model for neoplastic transformation was adapted for application to RR estimation in irradiated human populations. The model is rather complex (Scott 2006a,c) in that it involves different thresholds for activating and inhibiting the hormetic response in different humans. Low doses activate (stimulate) while moderate and high doses inhibit this response.
For low doses and dose rates that are associated with the hormetic zone (a low-dose zone with the lower end usually including elevated background radiation), a system of protective processes (PAM process along with a stimulated immune system, in addition to activated high-fidelity DNA repair) are considered to be maximally stimulated (Scott 2005, 2006a,b). For this dose zone, the RR at dose D (absorbed dose of all radiation involved) is given by the following:
| (1) |
The protection factor (PROFAC) gives the proportion of cancer cases (incidence or mortality) that are avoided among those cases that would otherwise have occurred spontaneously or in the absence of radiation hormesis. PROFAC, however, relates only to the low-LET component of the dose. Exposure to uranium ore dust and γ-rays may contribute 25–75% of the ‘effective’ dose to the lung in uranium mines. Applying this observation decreases the risk estimates of lung cancer by radon by a factor of 2-3, implicating no effect of radon and/or the presence of a threshold for lung cancer risk (Duport 2002). Thus, for radon exposures, PROFAC relates to the gamma-ray component of the radiation dose. High-LET alpha radiation by itself does not appear to activate the system of protective processes associated with radiation hormesis (Scott 2005, 2006b). However, more research on this topic is needed.
Thus, for exposure to low doses and dose rates of low-LET radiation or combinations of low- and high-LET radiations the dose-response curve is predicted to drop to a constant value given by 1 - PROFAC rather than increase as postulated by the widely used LNT hypothesis. However, there is a dose-rate dependent transition zone (Transition Zone A) where the individual-specific threshold for activating the protective hormesis process occurs (Scott 2005, 2006b). Over this zone, the dose-response curve is expected to progressively decrease below RR = 1 rather than suddenly drop to 1 - PROFAC. In any case, the lowest point of the dose-response curve can be quantified based on 1 - PROFAC. Further, this characterization of RR is expected to apply for most of the hormetic zone. Thus, where data are available for RR < 1 after a low dose, for low-dose-rate exposures to low or low plus high-LET radiations PROFAC can be justifiably estimated using:
| (2) |
Systematic error is expected to underestimate PROFAC when the equation is used for moderate and high doses, where a more complicated equation that depends on dose occurs and where hormesis can be suppressed. For doses in Transition Zone A, systematic error is also expected to favor underestimation of PROFAC. However, again, for a large portion of the hormetic zone, RR = 1 - PROFAC is expected to adequately characterize the dose-response relationship (Scott 2006a). The product 100 PROFAC allows representing the cancer cases avoided as a percentage (%) rather than as a proportion.
Standardized mortality ratios (SMR) can be used as estimates of RR, allowing PROFAC to be obtained based on SMR data. Odds ratio (OR) < 1 could also serve as an estimate of RR for the hormetic zone. However, for the unexposed group, irradiated persons should be excluded to the extent possible. Otherwise, a large systematic error could occur leading to changing a threshold-type hormetic curve into what appears to be a LNT curve. We have used both RR and SMR data to estimate PROFAC for a number irradiated human populations where hormetic effects have been demonstrated or suspected.
Values for PROFAC significantly > 0 imply that spontaneous cancers (possibly fatal) are being avoided that otherwise would have occurred in the absence of radiation hormesis. It should be kept in mind that what are considered spontaneous cancers may actually be associated with other environmentally associated risk factors. However, radiation hormesis would also be expected to protect against stochastic effects caused by such factors.
For a population residing in a high natural background radiation area where a significant component of the radiation dose is due to low-LET radiation, for each 100,000 spontaneous cancer deaths that would be expected over a given follow-up period in the absence of radiation hormesis, each increment of 0.1 in the PROFAC (due to radiation horme-sis) would be expected to save 10,000 lives. Thus, radiation hormesis is not a trivial benefit. Since many PROFACs reported later exceed 0.3, this translates into very large numbers of lives being saved due to radiation hormesis.
The PROFAC is considered to depend on dose rate, the type of radiation (i.e., radiation quality), and the target organ in the body. For moderate doses, more complicated dose-related terms arise and the RR cannot be adequately described using a single parameter, i.e., PROFAC. Thus, Equations 1 and 2 should be applied only to low doses and dose rates and only where the radiation exposure includes low-LET radiation. Presently, the same PROFAC is considered to apply to cancer incidence and cancer mortality (Scott 2006b).
Low-level Background Radiation and Hormesis
There are many epidemiological studies that demonstrate evidence for radiation hormesis (Luckey 1991), and Table 1 shows such studies for lung. The 13-yr U.S. Nuclear Shipyard Workers Study on the health effects of low-dose radiation was carried out by Johns Hopkins Department of Epidemiology (Matanoski 1991). In the study, a high-dose cohort of 27,872, low-dose cohort of 10,348, and a control cohort of 32,510 unexposed shipyard workers were examined from 1980–1988. Mortality from all causes was 24% less in nuclear workers than in non-nuclear workers. The SMR of 0.85 for all malignant tumors in the high-dose cohort was significantly less (p < 0.05) than in the control cohort; the PROFAC for lung cancer was 0.07.
TABLE 1.
Epidemiological studies demonstrating radiation hormesis in the lung
| PROFAC × 100 (%) | |||
| Study (workers/patients) | Reference | All Cancer | Lung Cancer |
| U.S.shipyard | Sponsler and Cameron 2005 | 16 | 7 |
| Airline crew | Blettner et al. 2002; Band et al. 1996 | 21–29 | 23–72 |
| U.S. nuclear | Howe el al. 2004 | 35 | 41 |
| Canadian nuclear | Zablotska et al. 2004 | 26 | 19–60 |
| Japanese nuclear | Iwasaki el al. 2003 | 6 | 28–57 |
| Korean nuclear | Jin et al. 2002 | 21–49 | 34 |
| UK nuclear | Carpenter et al. 1998 | 27 | 35–41 |
| UKAEA | Atkinson et al. 2004 | 10 | 11 |
| Russian nuclear | Khokryakov and Romanov 1994 | 7 | 35 |
| Canadian dose registry | Sont et al. 2001 | 21 | 31 |
| U.S. fluoroscopy | Howe 1995 | — | 6–18 |
| U.S. technologists | Doody etal. 1998 | — | 17–39 |
| Japanese technologists | Yoshinaga et al. 1999 | — | 38–55 |
| Indoor radon | Cohen 1995 | — | −20 |
| Japanese radon spa | Mifune 1992 | 37 | 45 |
| British radiologists | Berrington et al. 2001 | 29 | 26–100 |
| U.S. female nuclear | Wilkinson et al. 2000 | 17 | 47 |
| Breast cancer patients | Luckey 2003 | — | 50 |
| Mayak plutonium | Khokhriakov and Romanov 1996 | — | 47–61 |
Wilkinson and colleagues studied the causes of mortality for women working in 12 U.S. nuclear weapons facilities (Wilkinson et al. 2000). The study covered a total of 67,976 women who worked at these sites before 1980 and compared mortality data for those who wore radiation exposure monitoring badges with mortality in those workers who did not wear badges. A strong radiation hormesis effect was seen in all women in all facilities for all cancers. The study showed that there were 25 percent more deaths from all causes and 17 percent more deaths from cancer in unbadged workers than in those wearing badges. The RR for lung cancer mortality in unbadged women who were not monitored was 49 percent higher than in badged workers. Additionally, 10 of the 12 facilities showed decreased lung cancer frequency with PROFAC values ranging from 0.05 to 0.47 (Wilkinson et al. 2000).
Both the Shipyard and Wilkinson studies, which had appropriate internal controls for entrance into employment and medical care once employed, demonstrated clear evidence of radiation hormesis for lung cancer.
In a cohort of 45,468 Canadian nuclear power industry workers (1957–1994) the PROFAC for lung cancer was 0.19 in males and 0.60 in females (Zablotska et al. 2004). The PROFAC for lung cancers at the UK Chapelcross nuclear plant was 0.43 (p < 0.0001) based on Scottish rates and 0.35 when based on rates for England and Wales (McGeoghegan and Binks 2001). At three UK nuclear facilities, the SMR for lung cancer in workers with the highest cumulative whole-body dose (400+ mSv) was 0.59 for monitored workers and 0.97 for unmonitored workers (Carpenter et al., 1998). The PROFAC for lung cancer in United Kingdom Atomic Energy Authority (UKAEA) radiation workers was 0.11 compared to non-radiation workers (Atkinson et al. 2004).
Mortality was examined in 176,000 Japanese nuclear industry workers from 1986–1997. The PROFAC for lung cancer was 0.57 and 0.28 at cumulative doses of 50–100 mSv and > 100 mSv, respectively (Iwasaki et al. 2003). A strong radiation hormesis effect was observed at doses < 50 mSv (PROFAC = 0.49) and > 50 mSv (PROFAC = 0.56) for total cancer incidence in Korean nuclear workers (Ahn and Bae 2005). Of the 668 workers who died during 1984–1999, only 21 died of lung cancer, making the PROFAC for lung cancer 0.34 (Jin et al. 2002). Similarly, the PROFAC for lung cancer was 0.41 in a study of U.S. workers employed at 15 nuclear power plants between 1979 and 1997 (Howe et al. 2004).
Nearly 200,000 participants in the National Dose Registry of Canada from 1951–1988 were examined for cancer mortality. For lung cancer, the standardized incidence ratio (SIR) was 0.64 in males and 0.79 in females (Sont et al. 2001). Evidence of radiation hormesis (PROFAC = 0.13 at 0.01–0.49 Sv, PROFAC = 0.18 at 0.5–0.99 Sv, and PROFAC = 0.06 at 1.00–1.99 Sv) (Howe 1995) was found for lung cancer in fluoroscopy patients being treated for tuberculosis. Another study found a PROFAC of 0.20 at a cumulative lung dose of 0.84 Sv (Davis et al. 1989). Interestingly, in both studies the hormesis effect was similar in men and women (Davis et al. 1989; Howe 1995). The PROFAC for lung cancer in the contralateral lung at 10 or more years after diagnosis of breast cancer in those receiving fractionated radiotherapy was 0.50 at a lung dose of 1.4 Gy (Luckey 2003). And Prochazka et al. (2005) found increased lung cancer following radiotherapy for breast cancer only in smokers (RR = 2.0).
The PROFAC for lung cancer in German male and female aircraft cabin employees exposed to elevated solar and cosmic radiations was 0.43 (females) and 0.23 (males). In contrast the SMR for aircraft accidents ranged from 48–96 (Blettner et al. 2002). The SIR for lung cancer in pilots of Air Canada was 0.28, while the SMR for aircraft accidents was 26.6 (Band et al. 1996).
British radiologists who joined UK radiological societies between 1897 and 1979 were divided into four groups depending on when they joined: 1897–1920, 1921–1935, 1936–1954, and 1955–1979. Exposure limits during 1936–1954 were 2 mSv d−1 or 500 mSv yr−1, and 50 mSv yr−1 from 1955–1979. SMR comparisons were made with UK male non-radiology physicians (Table 2). The PROFAC for all cancers was 0.29 in the 1955–1979 cohort, while the PROFAC for lung cancer was 0.26 in the 1936–1954 cohort and 1.0 for the 1955–1979 cohort (6.5 cases were expected and none observed) (Berrington et al. 2001). The PROFAC for all cancer for all age-matched men was 0.54. For all cohorts post 1920 the PROFAC for lung cancer was 0.30 (Berrington et al. 2001). The PROFAC for lung cancer in Japanese technologists was 0.38 for those who worked from 1897–1933 and 0.55 for those who worked from 1934–1950 (Yoshinaga et al. 1999). The PROFAC values for lung cancer in U.S. technologists who worked from 1926–1939, 1940–1949, 1950–1959, 1960–1982 were 0.28, 0.24, 0.17, and 0.39, respectively (Doody et al. 1998).
TABLE 2.
Lung cancer mortality in British radiologists from 1897–1979*
| SMR (COMPARED TO UK MALE NON-RADIOLOGIST PHYSICIANS) | |||
| Years Joined Radiological Societies | Tolerance or Exposure Limits | All Cancers | Lung Cancer |
| 1897–1920 | > 1 Sv yr1 | 1.75 | 2.46 |
| 1921–1935 | < 1 Sv yr1 | 1.24 | 1.06 |
| 1936–1954 | 2 mSv day−1 or 500 mSv yr−1 | 1.12 | 0.74 |
| 1955–1979 | 50 mSv yr−1 | 0.71 | 0 |
Recycled steel contaminated with cobalt-60 was used in the construction of more than 180 buildings in Taiwan that housed 10,000 persons for a 9-–20-yr period. The average whole-body dose for these residents was 0.4 Sv given at an average dose-rate of 50 mSv/yr. Of the approximately 1,100 persons who received cumulative doses of 4 Sv from 1983–2003,only seven cases of fatal cancer were found. The cancer mortality rate of residents was 3.5 per 100,000 person-years, while the expected spontaneous cancer mortality rate was 116 persons per 100,000 person-years, giving residents a PROFAC 0.97 for fatal cancer. Yet the LNT hypothesis used by the International Commission on Radiological Protection (ICRP) predicted 302 cancer deaths in the Taiwan study –– 232 spontaneous and 70 caused by radiation (Chen et al. 2004).
A PROFAC for lung cancer of 0.35 was found at a dose-range of 0.26–1.0 Sv in Russian nuclear radiochemical workers at Mayak (Khokhryakov and Romanov 1992, 1994). The workers had mean lung doses that ranged from 1–2 Gy, and nearly all lung cancer cases were found in smokers. A linear fit for a lung cancer and smoking index has been employed for Mayak workers (Tokarskaya et al. 1995, 1997; Jacob et al. 2005). Estimates of radiation-induced lung cancer have been greatly overestimated when not adjusting for cigarette smoking. A RR = 0.91 for lung cancer was found in Mayak workers with lung gamma-ray doses > 2 Gy (Tokarskaya et al. 2002).
A Mayak case-control study of all morphologically verifiable lung cancer cases from 1966–1991 among the Mayak nuclear workers found a threshold of 3.7 kBq or 0.80 Gy for incorporated 239Pu. The incidence of lung cancer at lung doses < 0.8 Gy was significantly less (p < 0.05) than control levels (PROFACs of 0.44, 0.41, and 0.17 at average 239Pu body burdens of 0.34 kBq, 1.2 kBq, and 4.2 kBq, respectively) (Tokarskaya et al. 1997). A later analysis indicated a threshold of 1 Gy (20 Sv) and a curvilinear relationship between lung cancer and alpha radiation dose (Tokarskaya et al. 2002). The PROFAC for lung cancer among Mayak workers from chronic alpha plus gamma irradiation was 0.61 at a lung dose of 0.1–12 mGy and 0.47 for a dose of 12.1–50 mGy (Khokhriakov and Romanov 1996). A recent analysis using a more complicated form of the HRR model, which included an alpha-radiation dose in addition to the PROFAC, yields an estimate of 0.86 for the PROFAC (Scott 2006b).
The results obtained indicated that not only did hormesis-associated protective processes prevent spontaneous cancer but also reduced the number of lung cancers associated with alpha irradiation and cigarette smoking by the same factor, i.e., an 86% reduction. Thus, radiation hormesis appears to not only protect against spontaneous lung cancer but also against lung cancers associated with cigarette smoking and exposure to chronic alpha and gamma irradiation.
A study of Rocky Flats Plant employees who worked from 1951–1989 failed to demonstrate a dose-response in lung cancer incidence. Only by using restrictive regression analyses could an increased lung cancer rate be shown (Brown et al. 2004). Other studies showed a PROFAC for lung cancer mortality in plutonium workers at the Rocky Flats Plant of 0.86 (one observed, seven expected) (Tietjen 1987), and Voelz et al. (1983) found a PROFAC 0.80 for lung cancer in these plutonium workers. Manhattan Project plutonium workers had a median internal dose of 1.25 Sv and in all causes of death a PROFAC of 0.57 compared to the general population; their PROFAC for lung cancer was 0.32 (Voelz et al. 1997).
A cohort of 4402 workers at the Mound Facility were chronically exposed to 210Po from 1944–1972 but no dose-response trend was observed. When a 10-yr latency period was considered, the PROFACs for lung cancer at the two highest dose groups were 0.46 and 0.66 (Wiggs et al. 1991). UK workers at the Springfields uranium fuel fabrication facility had a median cumulative dose of 9.3 mSv and a PROFAC of <1 for lung cancer, while UK 235U enrichment workers at the Capenhurst facility had a median cumulative dose of 1.7 mSv and a PROFAC of <1 (McGeoghegan and Binks 2000a,b).
RADON
Radon exposure involves both high-LET alpha and low-LET gamma radiations. The gamma ray component is considered to stimulate hormet-ic effects (Scott 2005, 2006a,b). The beneficial effects of inhaled radon and radon-laden water are evident in Russian and European spa hospitals where hundreds of thousands of patients are annually treated for a variety of inflammatory, immune, and hormonal disorders at radon concentrations up to a 1000 times that of the U.S. Environmental Protection Agency (USEPA) residential radon limit (Deetjen and Falkenbach 1999; Mitsunobu et al. 2003; Yamaoka et al. 2005). Protracted low-dose irradiation with radon enhances cell-mediated immunity and reduces pulmonary metastasis of melanoma in mice (Takahashi and Kojima 2006). Radon balneology (therapeutic effects of baths) has been shown to be effective in randomized double-blind studies (Franke et al. 2000). Bogoljubov (1988) found an optimum therapeutic dose for radon of 2 mSv given over a 2-wk period. Interestingly, no increase in lung cancer has been found in radon spa never smoking patients or residents living in nearby high background radiation areas.
Normal background radiation exposures are mainly in the range of 2.5–4.0 mSv yr −1. However, they can exceed 10 times these values in various parts of the world (Wei 1997). More than half (∼ 2 mSv yr−1) of the United States' natural background radiation is associated with radon (mostly 222Rn) and its daughter radionuclides. About 250,000 Americans, living in a handful of Rocky Mountain states where lung cancer rates are much lower than predicted by the USEPA using the LNT hypothesis, receive background exposures of ∼ 40 mSv yr−1 (ACS 1997; Jagger 1998). Relative to other states, Colorado has the third lowest lung cancer death rate in the nation. For the period 1993–1997, the Colorado cancer death rate per 100,000 population was 48.2 for males and 25.6 for females. These rates are well below the national averages of 69.4 for males and 34.0 for females. Colorado radon levels are well above the national average, averaging 7.3 pCi/L. The USEPA estimates the average indoor radon level nationwide is 1.3 pCi/L (City of Fort Collins 2005). The relative rates of cancer in most native populations of Iran, India, and China that are exposed to high levels of background radiation have, in most cases, appeared to decrease (Wei 1997; NCRP 2001). Residents near Yangjiang in Guangdong province, China, receive an annual background dose of 6.4 mSv. The PROFAC was 0.19, while the ERR per Sievert for lung cancer was −0.68 under the apparently inappropriate LNT assumption (Wei 1997; Wei and Sugahara 2000). Use of the LNT function seems inappropriate in that a PROFAC > 0 implicates a hormetic dose-response.
According to the Biological Effects of Ionizing Radiation Report (BEIR) VI, the estimated annual number of radon-related lung cancers in never smokers in the United States ranges from 2,100–2,900 (NRC 1999). The National Research Council (NRC) found that exposure to radon in underground mines is associated with an increased risk of lung cancer (NRC 1999). Although a large fraction of uranium miners were smokers and smoking-related small-cell carcinoma represents the majority of lung cancer cases in American uranium miners (NRC 1999; Saccomanno et al. 1996), the RR for lung cancer was ∼ 25–29 in uranium miners with cumulative exposures of > 1,450 WLM† compared to those exposed to < 80 WLM (Gilliland et al. 2000; Lubin et al. 1995). One Working Level Month (WLM) corresponds to an exposure to one Working Level (WL) for 170 hours. One WL is equivalent to the potential alpha energy present in 100 pCi L−1 or 3,700 Bqm-3 of 222Rn in equilibrium with its short-lived decay products. Most cohort studies of uranium miners used the LNT hypothesis to estimate lung cancer risk (Lubin and Boice 1997; NRC 1999). The ERR per WLM in never smokers was about three times higher than in smokers (Lubin et al. 1995). A study of Australian uranium miners showed an increased lung cancer risk at exposures > 40 WLM (Woodward et al. 1991). A case-control study of WISMUT company uranium miners in Germany did not show a statistically significant increase for the incidence of lung cancer until cumulative exposures of > 800 WLM (Bruske-Hohlfeld et al. 2006).
The combined effect of indoor radon and smoking has not been clarified. The ability of indoor radon to cause lung cancer in never smokers is unclear (Krewski et al. 1999; Neuberger and Gesell 2002; Neuberger and Field 2003). Nearly all individual epidemiological studies of lung cancer from indoor radon fail to demonstrate a clear dose-response relationship, which is the strongest criteria for inferring causation. Essentially, all studies of indoor radon fail to find a significant association with lung cancer in never smokers (Krewski et al. 1999; Neuberger and Gesell 2002; Darby et al. 2005). No significant risk of lung cancer was found in never smokers in two large meta-analysis studies of indoor radon (Lubin and Boice 1997; Darby et al. 2005). Neuberger and Field (2003) reviewed the role of radon in the IARC (International Agency for Research on Cancer) database and could not find a single study that found it to be an occupational risk factor for lung cancer in never smokers. Detailed data on smoking status and dose groupings was not given in a number of studies (Neuberger and Gesell 2002).
Radon Exposure and Relative Risk
Several case-control epidemiological studies have investigated the relationship of radon exposure and lung cancer, presenting risk as ERR using the LNT hypothesis (Kreuzer et al. 2003); most of the studies show a positive ERR using the LNT hypothesis. However, some studies showed a negative ERR (Blot et al. 1990; Letourneau et al. 1994; Kreienbrock et al. 2001). In a report on indoor radon and lung cancer in France, Baysson et al. (2004) claimed a small ERR. The data was compatible with a decreasing risk rather than an increasing risk (Lachet 2005). A well designed case-control study in Finland over a large range of indoor radon concentrations (50–1277 Bq/m3 or 1.4–34.5 pCi/L) failed to show an increase in lung cancer in smokers (Auvinen et al. 1996). The Iowa radon lung cancer study, using ERR estimates, claims a non-significant increase in lung cancer in never smokers at 5–19 WLM. However, it does not present data on the various dose groupings from which the estimate was constructed. But Field et al. (2000) found there were also significant differences in demographic characteristics between cases and controls (Field et al. 2000).
Meta-analyses of case-control studies of lung cancer risk and radon exposure failed to provide relative risk data for dose-exposure groups in each study, giving only the excess odds ratio at 100 Bq/m3 obtained by regression using the LNT hypothesis (Krewski et al. 1999, 2005; Darby et al. 2005). A meta-analysis of eight case-control studies of indoor radon and lung cancer showed significant differences between the studies in exposure-response relationships (Lubin and Boice 1997), and five of the studies showed evidence of hormesis (Kauffman 2003). In the Shenyang, China, study, a PROFAC of 0.25 was found at 200–249 Bq/m3 and no risk of lung cancer in never smokers was found at any radon dose (Lubin et al. 2004).
Cohen's study encompassed about 300,000 radon measurements in 1,601 counties of the United States, representing about 90% of the residents living there (Cohen 1995). The trend of county lung cancer mortality was strikingly negative even after adjusting for smoking and many other socioeconomic factors. Using ecologic epidemiological studies, Cohen found a consistent, strong inverse relationship between indoor radon and lung cancer in non-smoking males and females as well as for smoking males and females (Cohen 1987, 1990, 1993, 1995). For lung cancer in males the slope was −7.3 %/pCiL−1 for 1970–1979 data and −7.7 %/pCiL−1 for the 1979–1994 data (Cohen 2000b). Equally strong negative correlations with radon exposure were also found for oral and laryn-geal cancers associated with smoking (Puskin 2003). Cohen's data points have very small error bars which are given for small increments of dose. Cohen later evaluated over 500 confounding issues using a wide variety of tests and models (Cohen 1995, Cohen 2000). Testing of a BEIR-VI proposal (NRC 1999) failed to explain the negative association of lung cancer with radon dose (Cohen 1999). Nothing explained the large negative correlation of lung cancer with increasing radon exposure. A lower absolute oxygen concentration in inspired air at higher altitudes was postulated as the cause of fewer lung cancers (Van Pelt 2003). An increase in red blood cell production stimulated by renal release of erythropoietin in response to reduced oxygen levels at high altitudes would normalize tissue oxygen levels to those at sea level (Huff et al. 1951). At higher lung doses the radiation hormesis effect disappears and the lung cancer risk reaches the expected spontaneous incidence.
Numerous reports have shown a negative relationship between environmental radon levels and lung cancer rates and a threshold (Bowie and Bowie 1991; Neuberger 1992; Wang et al. 2002; Ghiassi-Nejad and Mortazavi 2005; Mortazavi et al. 2006). A case-control study in Finland gave results on lung cancer similar to that of Cohen (Auvenin et al. 1996). Haynes evaluated 55 counties in England and Wales and also found a statistically significant negative association between radon concentration and lung cancer (Haynes 1988). Furthermore, a Swedish study suggested a PROFAC of 0.10 in smokers exposed to indoor radon, yet no increased lung cancer was seen in nonsmokers at doses >10.8 pCi/l (Pershagen et al. 1994). The PROFAC for lung cancer in female non-smokers in four counties of Saxony in East Germany was 0.40. The average indoor radon levels exceeded the country average by 3- to 10-fold (Arndt 1992). Overall RR of lung cancer in females in the Free State of Saxony was 0.98 for radon levels of < 100 500 Bq/m3 (Kaletsch et al. 1999). A fewer number of lung cancers than expected were found in the northeast Hungarian village of Matraderecske, which has a radon level range of 110–165 Bq/m3 (Toth et al. 1998). In Western Germany the PROFAC was 0.19 at > 140 Bq/m3 with an ERR of −0.12, but the ERR was −0.02 when adjusted for smoking and asbestos (Kreienbrock et al. 2001). The RR of lung cancer in a radon spa area of Japan was significantly less than in controls with PRO-FAC values of 0.45; for all cancers the PROFAC was 0.33 (Little 2002).
DISCUSSION
Socioeconomic status, diet and exercise may play an important role in lung cancer risk (Sanders 1996; Mattson 2005; Mao et al 2001; Faggiano et al 1997). Cancer incidence varies widely among countries of the world with inter- and intra-country incidences of site-specific cancers varying by over 100-fold, correlating with a wide range of dietary and lifestyle variables (Singletary 2000; Parkin et al. 2003). At least 25 classes of phyto-chemicals have been shown to exert significant anticarcinogenic activity in animals and humans (Moon and Mehta 1989; Roe 1994; Sanders 1996; Hecht 1999; Feinberg et al. 2002). Numerous studies have shown that exercise and dietary caloric restriction decrease tumorigenesis and enhance antioxidant compounds and immune defenses (Kohl et al. 1988; Roe 1990; Paffenbarger et al. 1992; Sanders 1996; Masoro 2005).
Environmental exposure to side-stream cigarette smoke is a major indoor air pollutant that poses a significant health hazard to never smokers (Wu-Williams et al. 1990). Lung cancer deaths from passive cigarette smoke exposure account for approximately a quarter of lung cancers seen in never smokers (Janerich et al. 1990). The RR of lung cancer in married never smokers varies up to 3.4, increasing with increased spousal cigarette consumption (USDHHS 1986; Fontham et al. 1994).
Cigarette smoke is in itself a complex mixture of chemicals that syner-gistically interacts with ethanol to induce oral/pharyngeal cancers (Blot 1992) and with asbestos to induce lung cancer (Hammond et al. 1979). RR values for lung cancer in asbestos workers, smokers, and asbestos workers who smoked were 5.2, 11, and 54, respectively (Hammond et al. 1979). Lung cancer in humans is also associated with exposures to a variety of other chemical agents (Sanders 1986; Williams and Sandler 2001). The RR for lung cancer for residents in urban areas of high air pollution is about 1.5 times greater than for people living in rural areas (Lave and Seskin 1970; Jedrychowski et al. 1990). The BEIR VII report (NRC 2005) and radioprotection agencies have not considered the role of many positive and negative confounding factors in determining risk estimates of lung cancer from exposure to ionizing radiation.
The cancer burden from tobacco use is enormous, confounding the often much smaller lung cancer risks associated with ionizing radiation. Useful assessments of radiation risk require accurate estimation of active and passive cigarette smoking exposures. The synergistic interaction of smoking and high-level radiation makes cancer estimation from high-level radiation even more difficult (Tokarskaya et al. 2002). Because of this synergism, passive cigarette smoke exposure in never smokers may also enhance the risk of radiation effects in the lung. Studies in never smokers to delineate the effects of passive smoke exposure and radiation need to be carried out; but there is no evidence for radiation risk of lung cancer in never smokers at doses < 1–2 Gy. If the interaction between radiation and smoking is more than additive, then the radiation-related risk of lung cancer would be greater in smokers than in never smokers. Nuclear facilities, in addition to prohibiting smoking at the work site should also educate their workers on the synergistic effects of smoking and radiation.
In their review of the literature, Rossi and Zaider (1997) said: “A critical review of the literature leads to the conclusion that at the radiation doses generally of concern in radiation protection (< 2 Gy), protracted exposure to low linear-energy transfer (LET) radiation (x- or gamma rays) does not appear to cause lung cancer”. Our conclusions are similar and extended to < 1 Gy doses in the lung from protracted high LET alpha irradiation from inhaled radon and 239Pu in combination with protracted low-LET gamma rays. The low-LET radiation component (e.g., gamma rays as is associated with radon and gamma-ray exposure of Mayak workers) is thought to trigger the hormetic response and protect against stochastic effects of the alpha radiation dose (Scott 2005, 2006b).
The BEIR VI committee estimated that 10–15% of the annual 160,000 lung cancer deaths in the United States may be attributed to indoor radon, with an uncertainty of 3,300–32,000 deaths (Krewski et al 1999; NRC 1999). The committee used lung cancer data from 11 major studies of underground miners, who are the most heavily exposed to radon, to estimate the risk of lung cancer by radon in residences (NRC 1999). The mean cumulative exposure among miners was about 30-fold higher than that found in an average home. The BEIR VI committee felt that it is especially difficult to estimate radon risks for never smokers in homes using high-dose data from uranium miners; “Most of the radon-related deaths among smokers would have not occurred if the victims had not smoked” (NRC 1999). The committee also felt that the assumption of linearity of risk down to the lowest exposures could not be validated against observational data due to the possible presence of a threshold (NRC 1999).
The healthy worker effect (HWE) is the name given to a common observation of employee cohorts that show a reduced mortality from all causes and/or cancer than those in the general population. The HWE has been attributed to pre-employment medical screening examinations, better working and socioeconomic conditions, and superior medical care for nuclear facility personnel. The HWE is negated by epidemiological studies of cancer mortality rates in exposed and unexposed workers in the same plant (Table 3) (Luckey 1991, 2003; Mortazavi and Ikushima 2006). The radiation workers were compared in several studies with non-radiation workers in the same plant, facility, or environment in order to decrease the biases associated with the HWE. The HWE is usually weaker for mortality from all cancers than for all causes of death (Baillargeon 2001). No reduction in mortality from all cancers was found in men who received annual medical physicals compared to men who did not (Friedman et al. 1986; Franks et al. 1996). The HWE does not explain radiation hormesis responses found in epidemiological studies that do not include workers. Epidemiological studies that compare exposed and unexposed cohorts in the same company or workplace, where medical procedures for employment and employee health are similar, should best delineate the HWE from hormesis (Luckey 1991). The large size of the Nuclear Shipyard Worker Study and Wilkerson multi-facility studies for exposed and control cohorts provided powerful, statistically significant evidence for radiation hormesis (Matanoski 1991; Wilkinson et al. 2000). The PROFAC of 0.29 for all cancers in British radiologists compared to all other physicians' specialties also excludes the HWE (Berrington et al. 2001; Cameron 2002). These studies, which had appropriate internal controls for entrance into employment and medical care once employed, demonstrated clear evidence of radiation hormesis and not a HWE. The rather ubiquitous nature of HWE-like radiation hormesis responses in cellular, animal, and epidemiological studies would also negate the HWE as an explanation for the radiation hormesis phenomenon in human population studies (Sanders 2006).
TABLE 3.
Epidemiological studies with internal controls that negate the HWE*
| Worker Comparison | Reference | PROFAC × 100 Lung Cancer (%) |
| Badged/unbadged U.S. DOE female workers | Wilkinson el al. 2000 | 49 |
| Radiologists/physicians, UK | Berrington el al. 2001 | 26–100 |
| High-dose/control shipyard workers | Sponsler and Cameron 2005 | 7 |
| Monitored/unmonitored UK nuclear workers | Carpenter el al. 1998 | 39 |
| Radiation/non-radiation UKAEA nuclear workers | Atkinson el al. 2004 | 11 |
| Radiation/non-radiation UK nuclear workers | McGeoghegan and Binks 2000a,b; 2001 | 3–43 |
In each study, radiation-exposed cohorts were compared with non-radiation cohorts in the same workplace or environment to reduce the biases resulting in the HWE.
With the LNT hypothesis, any dose of radiation is expected to increase the risk of lung cancer. In using the LNT hypothesis, BEIR VII, ICRP, USEPA, and National Council on Radiation Protection and Measurements (NCRP) ignore the possible presence of any threshold. Although admitting that simple extrapolation from high doses may not be justified, they feel that it is scientifically justified to do so (Sanders 2006). In stark contrast, the 2005 French Academy of Sciences (Paris) and the National Academy of Medicine report (Aurengo et al. 2005) concluded that the LNT hypothesis should not be used for low-LET doses < 100 mGy and especially not for doses < 10 mGy for assessing carcinogenic risks (Tubiana and Aurengo 2005). The French Academies found abundant evidence for radiation hormesis and believed that this data should be implemented in making radiation protection guidelines (Aurengo et al. 2005). “No one has been identifiably injured by radiation while working within the first numerical standards set first by the NCRP and then the ICRP in 1934” (Taylor 1980). The 1934 ICRP standard was about 500 mSv yr−1 (Cameron 2002).
The LNT hypothesis is widely used for estimating cancer risk, even though it has not been validated by scientific study and is not consistent with radiobiological data (Calabrese and Baldwin 2000; Tubiana 2003; Aurengo et al. 2005). Comparatively little thought has been given by the BEIR committees and the ICRP to hormesis associated with the radiation adaptive response and thresholds at low doses and low dose-rates (Feinendegen et al. 1988; Shadley and Wiencke 1989; NRC 1999, 2005; ICRP 2004), yet the BEIR VII report and the ICRP continue to support the LNT hypothesis (ICRP 2004). Because the LNT hypothesis is very well established and because many strong radiation protection organizations are in place, scientists and government officials are very reluctant to seriously consider the implications of the radiation hormesis phenomenon, which has obvious important health implications (Chen et al. 2004).
The current biased practice by some who report epidemiological results is misleading. Meta-analyses or individual epidemiological publications should provide the cancer risks for all dose categories, including the lowest doses for each study, and not just a single ERR estimate of cancer obtained by the LNT hypothesis. The practice of grouping several low-dose categories into one dose group to remove evidence of radiation hormesis should be abandoned. Estimates of excess lung cancer risk at exposures less than threshold values (such as < 800 WLM in German miners (Bruske-Hohlfeld et al. 2006) are not credible. The LNT hypothesis should clearly not be used when not appropriate in order to best present the true dose-response relationship of epidemiological data.
Cells carry in their genome a program for self-destruction called apoptosis. Apoptosis limits the accumulation of potentially harmful cells. Failure to regulate tissue homeostasis can result in cancer development (Thompson et al. 1992). Cell regulators, such as growth factors, cytokines, and hormones, are involved with the activation and repression of genes associated with apoptosis. Apoptosis is enhanced by low to moderate doses of ionizing radiation in normal tissues and tumors (Stephens et al. 1991). A low-dose PAM process, limiting potential cancer formation, may be activated by low-dose, low-LET gamma or x-radiations (Scott 2006a,b,c). Low-dose radiation may stimulate apoptosis of cells transformed by chemical carcinogens (Mitchel et al. 1999; Sakai et al. 2003). Furthermore, low dose ionizing radiation may enhance the elimination by apoptosis of cigarette-smoke-induced transformed pulmonary cells, thus decreasing lung cancer risk (Scott 2006b).
ACKNOWLEDGMENTS
The authors greatly appreciate graphics done by Sukwhun Sohn, Ph.D. candidate, and the support of Professors Hee Cheon No and Gyuseong Cho of the Nuclear and Quantum Engineering Department, KAIST, Daejeon, Republic of Korea. Bobby R. Scott thanks Jennifer Di Palma and Vicki Fisher of Lovelace Respiratory Research Institute for editorial assistance. The contribution of B.R. Scott to this work was supported by the Office of Science (BER), U.S. Department of Energy Grants DE-FG02-03ER63671 and DE-FG02-03ER63657.
Footnotes
WL, working level; WLM, working-level months; 1 WL = 3700 Bqm−3 = 100 pCiL−1; 1 WLM = 1 WL for 170 h = 5.1 mSv.
WL, working level; WLM, working-level months; 1 WL = 3700 Bqm−3 = 100 pCiL−1; 1 WLM = 1 WL for 170 h = 5.1 mSv.
REFERENCES
- ACS (American Cancer Society) Atlanta, GA: American Cancer Society; 1997. Cancer Facts & Figures –1997. [Google Scholar]
- Ahn Y and Bae J. 2005. A Chronic Exposure of Low-dose Radiation and Cancer Risks Among Nuclear Power Plant Workers in Korea. Proceedings of the Forty-Eighth Annual Meeting of the Japan Radiation Research Society and the First Asian Congress of Radiation Research, Abstract S11-2, p 89. Research Institute for Radiation Biology and Medicine, Hiroshima University, Japan
- ALA (American Lung Association). 2005. Trends in Lung Cancer Morbidity and Mortality. Part II. Graphs. American Lung Association, Epidemiology & Statistics Unit, Research Program Ser vices. Available at http://www.lungusa.org/atf/cf/%7B7A8D42C2-FCCA-4604-8ADE-7F5D5E762256%7D/lc2.pdf
- Ankathil R, Nair RK, and Padmavathi J, et. al. 2005. Review of Studies in High Level Natural Radiation Areas in India. Proceedings of the Forty-Eighth Annual Meeting of the Japan Radiation Research Society and the First Asian Congress of Radiation Research, Abstract S4-1-1, p 79. Research Institute for Radiation Biology and Medicine, Hiroshima University, Japan
- Arndt D. Die Strahlenexposition in den Bergbaugebieten Sachsens und Thüringens. In: Reiners Chr, et al., editors. Vol. 33. Stuttgart: Strahlenschutz in Forschung und Praxis; 1992. pp. 47–60. [Google Scholar]
- Atkinson WD, Law DV, Bromley KJ, et al. Mortality of employees of the United Kingdom Atomic Energy Authority, 1946–97. Occup Environ Med. 2004;61:577–585. doi: 10.1136/oem.2003.012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurengo A, Averbeck D, Bonnin A, et al. 2005. Dose-Effect Relationships and Estimation of the Carcinogenic Effects of Low Doses of Ionizing Radiation. Executive Summary. French Academy of Sciences, French National Academy of Medicine, Paris France
- Auvenin A, Maekelaeinen I, Hakama M, et al. Indoor radon exposure and risk of lung cancer: a nested case-control study in Finland. J Nat Cancer Inst. 1996;88:966–972. doi: 10.1093/jnci/88.14.966. [DOI] [PubMed] [Google Scholar]
- Baillargeon J. Characteristics of the healthy worker effect. Occup Med. 2001;16:359–366. [PubMed] [Google Scholar]
- Band PR, Le ND, Fang R, et al. Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Amer J Epidemiol. 1996;143:137–143. doi: 10.1093/oxfordjournals.aje.a008722. [DOI] [PubMed] [Google Scholar]
- Barros-Dios JM, Barreiro MA, Ruano-Ravina A, et al. Exposure to residential radon and lung cancer in Spain: a population-based case-control study. Amer J Epidemiol. 2002;156:548–555. doi: 10.1093/aje/kwf070. [DOI] [PubMed] [Google Scholar]
- Bauer S, Gusev BI, Pivina LM, et al. Radiation exposure due to local fallout from Soviet atmospheric nuclear weapons testing in Kazakhstan: solid cancer mortality in the Semipalatinsk historical cohort, 1960–1999. Radiat Res. 2005;164:409–419. doi: 10.1667/rr3423.1. [DOI] [PubMed] [Google Scholar]
- Baverstock K, Williams D. Chernobyl: an overlooked aspect? Letters. Science. 2002;299:44. doi: 10.1126/science.299.5603.44b. [DOI] [PubMed] [Google Scholar]
- Baysson H, Tirmarche M, Tymen G, et al. Indoor radon and lung cancer in France. Epidemiology. 2004;15:709–716. doi: 10.1097/01.ede.0000142150.60556.b8. [DOI] [PubMed] [Google Scholar]
- Berrington A, Darby SC, Weiss HA, et al. 100 years of observation on British radiologists: mortality from cancer and other causes 1987–1997. Brit J Radiol. 2001;74:507–519. doi: 10.1259/bjr.74.882.740507. [DOI] [PubMed] [Google Scholar]
- Blettner M, Zeeb H, Langner I, et al. Mortality from cancer and other causes among airline cabin attendants in Germany, 1960–1997. Amer J Epidemiol. 2002;156:556–565. doi: 10.1093/aje/kwf083. [DOI] [PubMed] [Google Scholar]
- Blot WJ. Alcohol and cancer. Cancer Res. 1992;52(Suppl)):2119s–2123s. [PubMed] [Google Scholar]
- Blot WJ, Xu Z-Y, Boice JD, et al. Indoor radon and lung cancer in China. J Natl Cancer Inst. 1990;82:10–25. doi: 10.1093/jnci/82.12.1025. [DOI] [PubMed] [Google Scholar]
- Bogoljubov WM. Clinical aspects of radon therapy in the USSR. Z Phys Med Balneol Med Klimatol. 1988;17:58–63. [Google Scholar]
- Bowie C, Bowie SHU. Radon and health. Lancet. 1991;337:409–413. doi: 10.1016/0140-6736(91)91177-v. [DOI] [PubMed] [Google Scholar]
- Brown SC, Schonbeck MF, McClure D, et al. Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: a case-control study. Amer J Epidemiol. 2004;160:163–172. doi: 10.1093/aje/kwh192. [DOI] [PubMed] [Google Scholar]
- Brown SO, Krise GM, Page HB. Continuous low-dose radiation effects on successive litters of albino rats. Radiat Res. 1963;19:270–276. [PubMed] [Google Scholar]
- Bruske-Hohlfeld I, Rosario AS, Wolke G., et al. Lung cancer risk among former uranium miners of the WISMUT company in Germany. Health Phys. 2006;90:208–216. doi: 10.1097/01.HP.0000175443.08832.84. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005;138:378–411. doi: 10.1016/j.envpol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exper Toxicol. 2000;19:41–75. doi: 10.1191/096032700678815602. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Scientific foundations of hormesis. Crit Rev Toxicol. 2001;31:351–624. doi: 10.1080/20014091111721. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Cameron JR. Correspondence: Radiation increased the longevity of British radiologists. Br J Radiol. 2002;75:637–639. doi: 10.1259/bjr.75.895.750637. [DOI] [PubMed] [Google Scholar]
- Caratero A, Courtade M, Bonnet L, et al. Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology. 1998;44:272–276. doi: 10.1159/000022024. [DOI] [PubMed] [Google Scholar]
- Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res. 1995;142:117–132. [PubMed] [Google Scholar]
- Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionizing radiation: retrospective cohort study in 15 countries. Brit Med J. 2005;331:77–80. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LM, Beral V, Smitm PG. Cancer mortality in relation to monitoring for radionu-clide exposure in three UK nuclear industry workforces. Brit J Cancer. 1998;78:1224–1232. doi: 10.1038/bjc.1998.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WL, Luan YC, Shieh MC, et al. Is chronic radiation an effective prophylaxis against cancer? J Amer Phys Surg. 2004;9:6–10. [Google Scholar]
- Chernobyl Forum. 2005. Chernobyl's Legacy: Health, Environmental and Socio-Economic Impacts. Chernobyl Forum Report 2003–2005, April 2005. International Atomic Energy Agency, Vienna, Austria
- City of Fort Collins, CO. 2005. Air Quality Department, available at http://www.ci.fort-collins.co.us/airqulaity/radon-health
- Cohen BL. Tests of the linear, no-threshold dose-response relationship for high-LET radiation. Health Phys. 1987;52:629–636. doi: 10.1097/00004032-198705000-00015. [DOI] [PubMed] [Google Scholar]
- Cohen BL. A test of the linear no-threshold theory of radiation carcinogenesis. Environ Res. 1990;53:193–220. doi: 10.1016/s0013-9351(05)80119-7. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Relationship between exposure to radon and various types of cancer. Health Phys. 1993;65(5):529–531. doi: 10.1097/00004032-199311000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Test of the linear no-threshold theory of radiation carcinogenesis for inhaled radon decay products. Health Phys. 1995;68:157–174. doi: 10.1097/00004032-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Testing a BEIR-VI suggestion for explaining the lung cancer vs. radon relationship for U.S. counties. Health Phys. 1999;78:522–527. doi: 10.1097/00004032-200005000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen BL. Updates and extensions to tests of the linear no-threshold theory. Technology. 2000a;7:657–672. [Google Scholar]
- Cohen BL. Cancer risk from low-level radiation. Amer J Roentgenol. 2002b;179:1137–1143. doi: 10.2214/ajr.179.5.1791137. [DOI] [PubMed] [Google Scholar]
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. Brit Med J. 2005;330:223–226. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F, Boice J, Hrubec Z, et al. Lung cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res. 1989;49:6130–6136. [PubMed] [Google Scholar]
- Deetjen P, Falkenbach A, editors. Frankfurt, Germany: P. Land; 1999. Radon und Gesundheit. [Google Scholar]
- Doll R. Cancer Res. Vol. 52. 1992. The lessons of life: keynote address to the nutrition and cancer conference; pp. 2024s–2029s. [PubMed] [Google Scholar]
- Doody MM, Mandel JS, Lubin JH, et al. Mortality among USA radiologic technologists 1926–1990. Cancer Causes Control. 1998;9:67–75. doi: 10.1023/a:1008801404245. [DOI] [PubMed] [Google Scholar]
- Duport P. Is the radon risk overestimated? Neglected doses in the estimation of the risk of lung cancer in uranium underground miners. Radiat Prot Dosim. 2002;98:329–338. doi: 10.1093/oxfordjournals.rpd.a006724. [DOI] [PubMed] [Google Scholar]
- Dupree EA, Watkins JP, Ingle JN, et al. Uranium dust exposure and lung cancer risk in four uranium processing operations. Epidemiol. 1995;6:370–375. doi: 10.1097/00001648-199507000-00007. [DOI] [PubMed] [Google Scholar]
- Faggiano F, Partanen T, Kogevinas M, et al. Socioeconomic differences in cancer incidence and mortality. IARC Sci Publ. 1997;138:65–176. [PubMed] [Google Scholar]
- Feinberg AP, Oshimura M, et al. Epigenetic mechanisms in human disease. Cancer Res. 2002;62:6784–6787. [PubMed] [Google Scholar]
- Feinendegen LE, Bond VP, Booz J, et al. Biochemical and cellular mechanisms of low-dose effects. Int J Radiat Biol. 1988;53:23–37. doi: 10.1080/09553008814550391. [DOI] [PubMed] [Google Scholar]
- Field RW, Steck DJ, Smith BJ, et al. Residential radon gas exposure and lung cancer. The Iowa radon lung cancer study. Amer J Epidemiol. 2000;151:1091–1102. doi: 10.1093/oxfordjournals.aje.a010153. [DOI] [PubMed] [Google Scholar]
- Fontham ETH, Correa P, Reynolds P, et al. Environmental tobacco smoke and lung cancer in nonsmoking women. J Amer Med Assoc. 1994;271:1752–1759. [PubMed] [Google Scholar]
- Franke A, Reiner L, Pratzel, et al. Long-term efficacy of radon spa therapy in rheumatoid arthri-tis-a randomized, sham-controlled study and follow-up. Rheumatology. 2000;39:894–902. doi: 10.1093/rheumatology/39.8.894. [DOI] [PubMed] [Google Scholar]
- Franks P, Gold MR, Clancy CM. Use of care and subsequent mortality: the importance of gender. Health Serv Res. 1996;31:347–363. [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Collen MF, Fireman BH. Multiphasic health checkup evaluation: a 16-year follow-up. J Chronic Dis. 1986;39:453–463. doi: 10.1016/0021-9681(86)90112-8. [DOI] [PubMed] [Google Scholar]
- Frigerio NA, Eckerman KF, and Stowe RS. 1973. Carcinogenic Hazard from Low-level, Low-rate Radiation, Part I. Report ANL/ES-26, Argonne National Laboratory, Argonne, IL
- Ghiassi-Nejad M and Mortazavi M. 2005. Radiation Adaptive Response Observed in Residents in High Level Natural Area of Ramsar. Proceedings of the Forty-Eighth Annual Meeting of the Japan Radiation Research Society and the First Asian Congress of Radiation Research, Abstract S4-2-2, p 81. Research Institute for Radiation Biology and Medicine, Hiroshima University, Japan
- Gilbert ES, Stovall M, Gospodarowicz M, et al. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat Res. 2003;159:161–173. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gilbert ES, Koshurnikova NA, Sokolnikov ME, et al. Lung cancer in Mayak workers. Radiat Res. 2004;162:505–516. doi: 10.1667/rr3259. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Hunt WC, Archer VE, et al. Radon progeny exposure and lung cancer risk among non-smoking uranium miners. Health Phys. 2000;79:365–372. doi: 10.1097/00004032-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Gray RG, Lafuma J, Parish SE, et al. 1986. Lung tumors and radon inhalation in over 2000 rats: approximate linearity across a wide range of doses and potentiation by tobacco smoke. In: Thompson RC and Mahaffey JA (eds), Life-Span Radiation Effects Studies in Animals: What Can They Tell Us? CONF-830951, NTIS, pp 592–607. Springfield, VA
- Hammond EC, Selikoff IJ, Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann NY Acad Sci. 1979;330:473–491. doi: 10.1111/j.1749-6632.1979.tb18749.x. [DOI] [PubMed] [Google Scholar]
- Hatch M, Ron E, Bouville A, et al. The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Epidemiol Rev. 2005;27:56–66. doi: 10.1093/epirev/mxi012. [DOI] [PubMed] [Google Scholar]
- Haynes RM. The distribution of domestic radon concentrations and lung cancer mortality in England and Wales. Rad Protect Dosim. 1988;25:93–96. [Google Scholar]
- Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768–774. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- Heidenreich WF, Luebeck EG, Hazelton WD, et al. Mutlistage models and the incidence of cancer in the cohort of atomic bomb survivors. Radiat Res. 2002;158:607–614. doi: 10.1667/0033-7587(2002)158[0607:mmatio]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Meinhardt TJ. Quantitative risk assessment of lung cancer in U.S. uranium miners. Health Phys. 1987;52:417–430. doi: 10.1097/00004032-198704000-00002. [DOI] [PubMed] [Google Scholar]
- Howe G.R. Lung cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with lung cancer mortality in the atomic bomb survivors study. Radiat Res. 1995;142:295–304. [PubMed] [Google Scholar]
- Howe GR, Zablotska LB, Fix JJ, et al. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004;162:517–526. doi: 10.1667/rr3258. [DOI] [PubMed] [Google Scholar]
- Huff RL, Lawrence JH, Siri WE, et al. Effects of changes in altitude on hematopoietic activity. Medicine. 1951;30(3):197–217. doi: 10.1097/00005792-195109000-00001. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection). 2004. Draft Report of Committee I/Task Group. Low Dose Extrapolation of Radiation Related Cancer Risk, Dec. 2004, Elsevier Science. Available at http://www.icrp.org/prod01.asp
- Iwasaki T, Murata M, Ohshima S, et al. Second analysis of nuclear industry workers in Japan, 1986–1997. Radiat Res. 2003;159:228–238. doi: 10.1667/0033-7587(2003)159[0228:saomon]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jacob V, Jacob P, Meckbach R, et al. Lung cancer in Mayak workers: interaction of smoking and plutonium exposure. Radiat Environ Biophys. 2005;44:119–129. doi: 10.1007/s00411-005-0012-5. [DOI] [PubMed] [Google Scholar]
- Jagger J. Natural background radiation and cancer death in Rocky Mountain states and Gulf Coast states. Health Phys. 1998;75:428–430. doi: 10.1097/00004032-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Janerich DT, Thompson WD, Varela LR, et al. Lung cancer and exposure to tobacco smoke in the household. N Engl J Med. 1990;323:632–636. doi: 10.1056/NEJM199009063231003. [DOI] [PubMed] [Google Scholar]
- Jaworowski Z. Stimulating effects of ionizing radiation: new issues for regulatory policy. Regul Toxicol Pharmacol. 1995;22:172–179. doi: 10.1006/rtph.1995.1082. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Becher H, Wahrendorf J, et al. A case-control study of lung cancer with special reference to the effect of air pollution in Poland. J Epidemiol Community Health. 1990;44:114–120. doi: 10.1136/jech.44.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YW, Jeong M, Sung SH, et al. Epidemiological investigation of deaths among radiation workers in nuclear power plants of Korea. J Korea Assoc Radiat Prot. 2002;27:233–237. [Google Scholar]
- Kaletsch U, Kaatsch P, Meinert R, et al. Childhood cancer and residential radon exposure- results of a population-based case-control study in Lower (Germany) Radiat Environ Biophys. 1999;38:211–215. doi: 10.1007/s004110050158. [DOI] [PubMed] [Google Scholar]
- Kant K, Chauhan RP, Sharma GS, et al. Hormesis in humans exposed to low-level ionizing radiation. Intern J Low Radiat. 2003;1:76–87. [Google Scholar]
- Kauffman JM. Radiation hormesis: demonstrated, deconstructed, denied, dismissed, and some implications for public policy. J Sci Explor. 2003;17:389–407. [Google Scholar]
- Khokhriakov VF, Romanov SA. Estimation of the temporal distribution and dose dependency of lung cancer among workers of nuclear fuel reprocessing plant. Health Phys. 1996;71:83–85. doi: 10.1097/00004032-199607000-00013. [DOI] [PubMed] [Google Scholar]
- Khokryakov VF, Romanov SA. Radiation impact on lung cancer. Nauchno-informatsionny byulleten yadernogo obshestva SSSR N. 1992;4:16–17. [Google Scholar]
- Khokryakov VF, Romanov SA. Lung cancer in radiochemical industry workers. Sci Total Environ. 1994;142:25–28. doi: 10.1016/0048-9697(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Kohl HW, LaPorte RE, Blair SN. Physical activity and cancer: an epidemiological perspective. Sports Med. 1988;6:222–237. doi: 10.2165/00007256-198806040-00004. [DOI] [PubMed] [Google Scholar]
- Kopecky KJ, Nakashima E, Yamamoto T, et al. 1986. Lung Cancer, Radiation, and Smoking among A-bomb Survivors, Hiroshima and Nagasaki. Report TR-13-86, Radiation Effects Research Foundation, Hiroshima, Japan
- Kostyuchenko VA, Krestina LYu. Long-term irradiation effects in the population evacuated from the East-Urals radioactive trace area. Sci Total Environ. 1994;142:119–125. doi: 10.1016/0048-9697(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kreienbrock L, Kreuzer M, Gerken M, et al. Case-control study on lung cancer and residential radon in Western Germany. Amer J Epidemiol. 2001;153:42–52. doi: 10.1093/aje/153.1.42. [DOI] [PubMed] [Google Scholar]
- Kreisheimer M, Koshurnikova NA, Nekolla E, et al. Lung cancer mortality among male nuclear workers of the Mayak facilities in the former Soviet Union. Radiat Res. 2000;154:3–11. doi: 10.1667/0033-7587(2000)154[0003:lcmamn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kreuzer M, Heinrich J, Wolke G, et al. Residential radon and risk of lung cancer in Eastern Germany. Epidemiology. 2003;14:559–568. doi: 10.1097/01.ede.0000071410.26053.c4. [DOI] [PubMed] [Google Scholar]
- Krewski D, Rai SN, Zielinski JM, et al. Characterization of uncertainty and variability in residential radon cancer risks. Ann NY Acad Sci. 1999;895:245–272. doi: 10.1111/j.1749-6632.1999.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology. 2005;16:137–145. doi: 10.1097/01.ede.0000152522.80261.e3. [DOI] [PubMed] [Google Scholar]
- Lachet B. Indoor radon and lung cancer. Letter to the Editor. Epidemiology. 2005;17:121. doi: 10.1097/01.ede.0000181632.92191.df. [DOI] [PubMed] [Google Scholar]
- Lave LB, Seskin EP. Air pollution and human health. Science. 1970;169:723–733. doi: 10.1126/science.169.3947.723. [DOI] [PubMed] [Google Scholar]
- Letourneau EG, Krewski D, Choi NW, et al. Case-control study of residential radon and lung cancer in Winnipeg, Manitoba, Canada. Amer J Epidemiol. 1994;140:310–322. doi: 10.1093/oxfordjournals.aje.a117253. [DOI] [PubMed] [Google Scholar]
- Little MP. Comparisons of lung tumour mortality risk in the Japanese A-bomb survivors and in the Colorado Plateau uranium miners: support for the ICRP lung model. Int J Radiat Biol. 2002;78:145–163. doi: 10.1080/09553000110095714. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Hollcroft JW, Miller E, et al. Long-term effects of acute and chronic radiation in mice. I. Survival and tumor incidence following acute irradiation of 0.11 r per day. J Natl Cancer Inst. 1955;15:1049. [PubMed] [Google Scholar]
- Lubin JH, Boice JD. Lung cancer risk from residential radon: meta-analysis of eight epi-demiological studies. J Natl Cancer Inst. 1997;89:49–57. doi: 10.1093/jnci/89.1.49. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Boice JD, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995;87:817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer. 2004;109:132–137. doi: 10.1002/ijc.11683. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Radiation Hormesis. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Luckey TD. A Rosetta stone for ionizing radiation. ROS Magazine. 2003;8:22–30. [Google Scholar]
- Mao Y, Hu J, Ugnat A-M, et al. Socioeconomic status and lung cancer risk in Canada. Int Epidemiol Assoc. 2001;30:809–817. doi: 10.1093/ije/30.4.809. [DOI] [PubMed] [Google Scholar]
- Martell EA. Tobacco radioactivity and cancer in smokers. Amer Sci. 1975;63:404–412. [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Matanoski GM. 1991. Health Effects of Low-level Radiation in Shipyard Workers. Final Report. Report No. DOE DE-AC02-79EV10095. U.S. Department of Energy, Washington, DC
- Mattson MP. Hormesis and disease resistance: Activation of cellular stress response pathways. BELLE Newsletter. 2005;13(3):6–14. doi: 10.1177/0960327107083417. Part 2. [DOI] [PubMed] [Google Scholar]
- McGeoghegan D, Binks K. The mortality and cancer morbidity experience of workers at the Springfields uranium production facility, 1946–95. J Radiol Prot. 2000a;20:111–137. doi: 10.1088/0952-4746/20/2/301. [DOI] [PubMed] [Google Scholar]
- McGeoghegan D, Binks K. The mortality and cancer morbidity experience of workers at the Capenhurst uranium enrichment facility 1946–95. J Radiol Prot. 2000b;20:381–401. doi: 10.1088/0952-4746/20/4/303. [DOI] [PubMed] [Google Scholar]
- McGeoghegan D, Binks K. The mortality and cancer morbidity experience of employees at the Chapelcross plant of British Nuclear Fuels plc, 1955–95. J Radiol Prot. 2001;21:221–250. doi: 10.1088/0952-4746/21/3/302. [DOI] [PubMed] [Google Scholar]
- Mifune M, Sobue T, Arimoto H, et al. Cancer mortality survey in a spa area (Misasa, Japan) with a high radon background. Jpn J Cancer Res. 1992;83:1–5. doi: 10.1111/j.1349-7006.1992.tb02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ, Gragtmans NJ, Morrison DP. Beta-radiation-induced resistance to MNNG initiation of papilloma but not carcinoma formation in mouse skin. Radiat Res. 1999;121:180–186. [PubMed] [Google Scholar]
- Mitsunobu F, Yamaoka K, Hanamoto K, et al. Elevation of antioxidant enzymes in the clinical effects of radon and thermal therapy for bronchial asthma. J Radiat Res. 2003;44:95–99. doi: 10.1269/jrr.44.95. [DOI] [PubMed] [Google Scholar]
- Monchaux G, Morlier JP, Morin M, et al. Carcinogenic and cocarcinogenic effects of radon and radon daughters in rats. Environ Health Perspect. 1994;102:64–73. doi: 10.1289/ehp.9410264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RC, Mehta RG. Chemoprevention of experimental carcinogenesis in animals. Prev Med. 1989;18:576–591. doi: 10.1016/0091-7435(89)90031-5. [DOI] [PubMed] [Google Scholar]
- Mortazavi SMJ, Ikushima T. Open questions regarding implications of radioadaptive response in the estimation of the risks of low-level exposures in nuclear workers. Int J Low Radiat. 2006;2:88–96. [Google Scholar]
- Mortazavi SMJ, Ghiassi-Nejad M, Karam PA, et al. Cancer incidence in areas with elevated levels of natural radiation. Int J Low Radiat. 2006;2:20–27. [Google Scholar]
- Nambi KSV, Soman SD. Environmental radiation and cancer in India. Health Phys. 1987;52:653–657. doi: 10.1097/00004032-198705000-00018. [DOI] [PubMed] [Google Scholar]
- NCRP (National Council on Radiation Protection and Measurements). 2001. Evaluation of the Linear-Nonthreshold Model for Ionizing Radiation. NCRP Report 136, National Council on Radiation Protection and Measurements, p. 6, Bethesda, MD
- NRC (National Research Council). 1999. Committee on Health Risks of Exposure to Radon, Health Effects of Exposure to Radon (BEIR VI), National Academy of Sciences, National Academy Press, Washington, DC.
- NRC (National Research Council). 2005. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. 2005. BEIR VII-Phase 2, Health Risks from Exposure to Low Levels of Ionizing Radiation, National Academy of Sciences, National Academy Press, Washington, DC [PubMed]
- Neuberger JS. Residential radon exposure and lung cancer: an overview of ongoing studies. Health Phys. 1992;63:503–509. doi: 10.1097/00004032-199211000-00001. [DOI] [PubMed] [Google Scholar]
- Neuberger JS, Field RW. Occupation and lung cancer in nonsmokers. Rev Environ Health. 2003;18:251–267. doi: 10.1515/reveh.2003.18.4.251. [DOI] [PubMed] [Google Scholar]
- Neuberger JS, Gesell TF. Residential radon exposure and lung cancer: Risk in nonsmokers. Health Phys. 2002;83:1–18. doi: 10.1097/00004032-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Lee I, Wing AL. The influence of physical activity on the incidence of site-specific cancers in college alumni. In: Jacobs MM, editor. Exercise, Calories, Fat, and Cancer. NY: Plenum Press; 1992. pp. 7–15. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Whelan, SL, Ferlay J, et al. 2003. Cancer incidence in five continents, Vol. VIII. IARC Scientific Publications No. 155, International Agency for Research on Cancer, Lyon, France
- Pershagen G, Akerblom G, Axelson O, et al. Residential radon exposure and lung cancer in Sweden. N Engl J Med. 1994;330(3):159–164. doi: 10.1056/NEJM199401203300302. [DOI] [PubMed] [Google Scholar]
- Pierce DA, Sharp GB, Mabuchi K. Joint effects of radiation and smoking on lung cancer risk among atomic bomb survivors. Radiat Res. 2003;159:511–520. doi: 10.1667/0033-7587(2003)159[0511:jeoras]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pierce DA, Shimizu Y, Preston DL, et al. Studies of the mortality of atomic bomb survivors. Report 12. 1. Cancer: 1950–1990. Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- Prochazka M, Hall P, Gagliardi G, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: Case-only design. J Clin Oncol. 2005;23:7467–7474. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- Puskin JS. Smoking as a confounder in ecologic correlations of cancer mortality rates with average county radon levels. Health Phys. 2003;84:526–532. doi: 10.1097/00004032-200304000-00012. [DOI] [PubMed] [Google Scholar]
- Redpath JL, Liang D, Taylor TH, et al. The shape of the of the dose-response curve for radiation-induced neoplastic transformation. Evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156:700–707. doi: 10.1667/0033-7587(2001)156[0700:tsotdr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Roe FJ. Diet is important in assessing lung cancer risk factors in nonsmokers. Nutr Cancer. 1994;22:203–206. doi: 10.1080/01635589409514346. [DOI] [PubMed] [Google Scholar]
- Roe FJC. 1200-rat biosure study: design and overview of results. In: Fishbein L, editor. Biological Effects of Dietary Restriction. Springer-Verlag, NY: ILSI Monographs; 1990. pp. 287–304. [Google Scholar]
- Rossi HH, Zaider M. Radiogenic lung cancer: The effects of low doses of low linear energy transfer (LET) radiation. Radiat Environ Biophys. 1997;36:85–88. [PubMed] [Google Scholar]
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in non-miners from 1947 to 1991. Cancer. 1996;77:1278–1283. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1278::AID-CNCR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hoshi Y, Nomura T, et al. Suppression of carcinogenic processes in mice by chronic low dose rat gamma-irradiation. Int J Low Radiat. 2003;1:142–146. [Google Scholar]
- Sanders CL. Columbus, OH: Battelle Press; 1986. Toxicological Aspects of Energy Production. [Google Scholar]
- Sanders CL. Prevention and Therapy of Cancer and Other Common Diseases: Alternative and Traditional Approaches. Richland, WA: Infomedix; 1996. pp. 44–51. [Google Scholar]
- Sanders CL. 2006. Hormesis as a confounding factor in epidemiological studies of radiation car-cinogenesis. J Korean Assoc Radiat Prot (in press)
- Schull WJ, Weiss KM. Radiation carcinogenesis in humans. Adv Radiat Biol. 1992;16:215–258. [Google Scholar]
- Scott BR. New Research on Genomic Instability (preliminary title) Nova Science Publishers, Inc.; 2006a. Radiation hormesis and the control of genomic instability. accepted chapter. [Google Scholar]
- Scott BR. 2006b. Low-dose radiation-induced protective process and implications for risk assessment, cancer prevention, and cancer therapy. Dose-Response (in press) [DOI] [PMC free article] [PubMed]
- Scott BR. Stochastic thresholds: A novel explanation of nonlinear dose-response relationships. Dose-Response. 2005;3:547–567. doi: 10.2203/dose-response.003.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Haque M, and Di Palma J. 2006. Biological basis for radiation hormesis in mammalian cellular communities Int J Low Radiat (in press)
- Sethi T. Science, medicine, and the future: lung cancer. Brit J Med. 1997;314:652–660. doi: 10.1136/bmj.314.7081.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989;56:107–118. doi: 10.1080/09553008914551231. [DOI] [PubMed] [Google Scholar]
- Shilnikova NS, Preston DL, Ron E, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159:787–798. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Singletary K. Diet, natural products and cancer chemoprevention. J Nutr. 2000;130:465S–466S. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- Sont WN, Zielinski JM, Ashmore AP, et al. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Amer J Epidemiol. 2001;153:309–318. doi: 10.1093/aje/153.4.309. [DOI] [PubMed] [Google Scholar]
- Spalding JF, Thomas RG and Tietjen GL. 1982. Life Span of C57 Mice as Influenced by Radiation Dose, Dose Rate, and Age at Exposure. Report LA-9528 (UC-48), Los Alamos National Laboratory, Los Alamos, NM
- Sponsler R, Cameron JR. Nuclear shipyard worker study (1980–1988): a large cohort exposed to low-dose-rate gamma radiation. Int J Low Radiat. 2005;1:463–478. [Google Scholar]
- Stephens LC, Kang K, Schultheiss TE, et al. Apoptosis in irradiated murine tumors. Radiat Res. 1991;127:308–316. [PubMed] [Google Scholar]
- Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res. 2006;165:337–342. doi: 10.1667/rr3501.1. [DOI] [PubMed] [Google Scholar]
- Taylor LS. Some non-scientific influences on radiation protection standards and practice. Health Phys. 1980;32:851–874. [PubMed] [Google Scholar]
- Thomas D, Pogoda J, Langholz JB, et al. temporal modifiers of the radon-smoking interaction. Health Phys. 1994;66:257–262. doi: 10.1097/00004032-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Strange R, Schedin PJ. Apoptosis in the genesis and prevention of cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:597–602. [PubMed] [Google Scholar]
- Tietjen GL. Plutonium and lung cancer. Health Phys. 1987;52:625–628. doi: 10.1097/00004032-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Tokarskaya EB, Okladnikova ND, Belyaeva ZD, et al. The influence of radiation and nonradiation factors on the lung cancer incidence among the workers of the nuclear enterprise “Mayak”. Health Phys. 1995;69:356–366. doi: 10.1097/00004032-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, et al. Multifactorial analysis of lung cancer dose-response relationships for workers at the Mayak nuclear enterprise. Health Phys. 1997;73:899–905. doi: 10.1097/00004032-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Tokarskaya ZB, Scott BR, Zhuntova GV, et al. Interaction of radiation and smoking in lung cancer induction among workers at the Mayak nuclear enterprise. Health Phys. 2002;83:833–846. doi: 10.1097/00004032-200212000-00011. [DOI] [PubMed] [Google Scholar]
- Toth E, Lazar I, Selmeczi D, et al. Lower cancer risk in medium high radon. Pathol Oncol Res. 1998;4:125–129. doi: 10.1007/BF02904706. [DOI] [PubMed] [Google Scholar]
- Tubiana M. The carcinogenic effect of low doses: the validity of the linear no-threshold relationship. Int J Low Radiat. 2003;1:1–33. [Google Scholar]
- Tubiana M, Aurengo A. Dose-effect relationship and estimation of the carcinogenic effect of low doses of ionizing radiation. Int J Low Radiat. 2005;2(3/4):134–151. [Google Scholar]
- USDHHS (U.S. Department of Health and Human Services). 1982. The Health Consequences of Smoking: Cancer. A Report of the Surgeon General. U.S. Public Health Service, DHHS Publication no. (PHS) 82-50179, Washington, DC
- USDHHS (U.S. Department of Health and Human Services). 1986. The Health Consequences of Involuntary Smoking. A Report of the Surgeon General. U.S. Public Health Service, Washington, DC
- USDHHS (Department of Health and Human Services). 1990. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. DHHS Publication No. (CDC) 90-8416, U.S. Public Health Service, Washington, DC
- USDHHS (U.S. Department of Health and Human Services). 2004. The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Public Health Service, Centers for Disease Control and Prevention, Washington, DC. Available at www.cdc.gov/tobacco/sgr/
- Van Pelt WR. Epidemiological associations among lung cancer, radon exposure and elevation above sea level-a reassessment of Cohen's county level radon study. Health Phys. 2003;85:397–40. doi: 10.1097/00004032-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Voelz GL, Wilkinson CS, Acquavelle JF. An update of epidemiologic studies of plutonium workers. Health Phys. 1983;44(Suppl 1):493–503. doi: 10.1097/00004032-198306001-00048. [DOI] [PubMed] [Google Scholar]
- Voelz GL, Lawrence JNP, Johnson ER. Fifty years of plutonium exposure to the Manhattan Project plutonium workers: an update. Health Phys. 1997;73:611–619. doi: 10.1097/00004032-199710000-00004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lubin JH, Wang L. Residential radon and lung cancer risk in a high-exposure area of Gansu Province, China. Amer J Epidemiol. 2002;155:554–564. doi: 10.1093/aje/155.6.554. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang W, Minamihisamatsu D, et al. 2005. Effect of Natural Radiation Compared with Those of Smoking and Air-Pollution. Proceedings of the Forty-Eighth Annual Meeting of the Japan Radiation Research Society and the First Asian Congress of Radiation Research, Abstract S4-1-3, p 80. Research Institute for Radiation Biology and Medicine, Hiroshima University, Japan
- Wei L. High background radiation area-an important source of exploring the health effects of low dose ionizing radiation. In: Wei L, Sugahara T, Tao Z, editors. High Levels of Natural Radiation, Radiation Dose and Health Effects. Amsterdam, The Netherlands: Elsevier; 1997. pp. 1–14. [Google Scholar]
- Wei L, Sugahara T. An introductory overview of the epidemiological study on the population at the high background radiation areas in Yangjiang, China. J Radiat Res. 2000;41(Suppl):1–7. doi: 10.1269/jrr.41.s1. (Toyko) [DOI] [PubMed] [Google Scholar]
- Wichmann HE, Rosario AS, Heid IM, et al. Increased lung cancer risk due to residential radon in a pooled and extended analysis of studies in Germany. Health Phys. 2005;88:71–79. doi: 10.1097/01.hp.0000142497.31627.86. [DOI] [PubMed] [Google Scholar]
- Wiggs LD, Cox-DeVore CA, Voelz G. Mortality among a cohort of workers monitored for 210Po exposure: 1944–1972. Health Phys. 1991;61:71–76. doi: 10.1097/00004032-199107000-00007. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Trieff N, Graham R, et al. 2000. Final Report. Study of Mortality Among Female Nuclear Weapons Workers. Grant Numbers: 1R01 OHO3274, R01/CCR214546, R01/CCR61 2934-01, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Atlanta, GA
- Williams MD, Sandler AB. The epidemiology of lung cancer. Cancer Treat Res. 2001;105:31–52. doi: 10.1007/978-1-4615-1589-0_2. [DOI] [PubMed] [Google Scholar]
- Wing S, Richardson D. Age at exposure to ionizing radiation and cancer mortality among Hanford workers: follow-up through 1994. Occup Environ Med. 2005;62:465–472. doi: 10.1136/oem.2005.019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward A, Roder D, McMichael AJ, et al. Radon daughter exposures at the Radium Hill uranium mine and lung cancer rates among former workers, 1952–87. Cancer Causes Control. 1991;2:213–220. doi: 10.1007/BF00052136. [DOI] [PubMed] [Google Scholar]
- Wu-Williams AH, Samet JM. Environmental tobacco smoke: exposure-response relationships in epidemiological studies. Risk Anal. 1990;10:39–48. doi: 10.1111/j.1539-6924.1990.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Sugie C, Futatsugawa S, et al. 2005. Basic Study of Biologic Effects of Thoron Hot Spring on Hypertension. Proceedings of the Forty-Eighth Annual Meeting of the Japan Radiation Research Society and the First Asian Congress of Radiation Research, Abstract P-A-108, p 139. Research Institute for Radiation Biology and Medicine, Hiroshima University, Japan
- Yiin JH, Schubauer-Berigan MK, Silver SR, et al. Risk of lung cancer and leukemia from exposure to ionizing radiation and potential confounders among workers at the Portsmouth Naval Shipyard. Radiat Res. 2005;163:603–613. doi: 10.1667/rr3373. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S, Aoyama T, Yoshimotot Y, et al. Cancer mortality among radiological technologists in Japan: updated analysis of follow-up data from 1969 to 1993. J Epidemiol. 1999;9:61–72. doi: 10.2188/jea.9.61. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S, Mabuchi K, Sigurdson AJ et al. 2003. Cancer Risks Among Radiologists and Radiologic Technologists: A Review of Epidemiological Studies. Annual Report 2002–2003, National Institute of Radiological Sciences, Chiba, Japan.
- Zablotska LB, Ashmore JP, Howe GR. Analysis of mortality among Canadian nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004;161:633–641. doi: 10.1667/rr3170. [DOI] [PubMed] [Google Scholar]
- Zahi S, Lin X, Pan T, et al. Report of survey on mortality from malignant tumors in high background area of Guangdong. Radiat Res. 1982;22:48. (Japan) [Google Scholar]