Abstract
Restraint elicits a number of physiological stress responses that can be increased or decreased in magnitude based on prior stress history. For instance, repeated exposure to restraint leads to habituation of hypothalamic-pituitary-adrenal (HPA) activation to restraint. In contrast, acute restraint after a different repeated stressor leads to facilitation of HPA activity to the novel stress. Acute restraint also elicits a variety of behaviors including struggling, but the effect of prior stress in regulating behavioral responses to restraint is not clear. The goal of the present studies was to assess struggling during restraint with or without a prior history of repeated stress. Using automated behavioral analysis software (EthoVision), we quantified struggling during restraint. We found that acutely restrained rats exhibit vigorous struggling behavior that declines during a single restraint period. Repeated restraint lead to habituated struggling behavior, whereas acute restraint after repeated swim elicited facilitated struggling behavior. These effects on struggling were found alongside expected differences in HPA activity. Removing stress-induced increases in corticosterone via adrenalectomy did not significantly affect struggling responses to restraint. Overall, restraint-induced struggling appears to be regulated in a manner similar to HPA responses to restraint, but is not dictated by adrenal hormones.
Keywords: restraint, struggling, ACTH, corticosterone, habituation, facilitation
Introduction
Restraint elicits a variety of physiological stress responses and the magnitude of these can be decreased or increased by prior stress history. Repeated restraint exposure leads to decreases in hypothalamic-pituitary-adrenal (HPA) activation and fos mRNA expression in stress-responsive brain areas to the familiar restraint, a phenomenon known as habituation [4, 10, 11, 16]. In contrast, in animals with a history of repeated experience with a different stressor, acute restraint can lead to facilitation, in which HPA and sympathetic activity meets or exceed the responses induced by naive exposure to acute restraint [3, 5, 6].
In addition to activating stress-sensitive physiological systems, acute restraint provokes a number of behaviors, including the production of fecal boli, ultrasonic vocalizations [20, 24]and struggling. In older literature, struggling during acute exposure to an immobilization paradigm was used to assess “irritability” during opiate withdrawal [15, 25]. More recently, it was shown that strains of rats bred for high or low levels of amygdala excitability and seizure kindling exhibit high or low levels of struggling, respectively, during acute immobilization [2, 17, 18]. This difference in struggling paralleled between-strain differences in HPA responses to acute immobilization. Higher levels of struggling during acute immobilization are also associated with an increased incidence of gastric ulcers [14, 27]. This evidence suggests that the degree of struggling elicited by restraint or immobilization may itself be a useful measure of the stress response, and may parallel other indices of stress. However, the above studies focused only on acute exposure to restraint or immobilization and measurements of struggling were not well-defined or quantified. If struggling to restraint does parallel other indices of the stress response in restrained animals, it should be predictably changed in magnitude depending on prior stress history. Therefore, the goal of the present studies was to measure struggling behavior and determine whether it is modified under conditions that typically produce habituated and facilitated HPA responses to restraint, and determine whether the degree of struggling observed follows the same pattern as HPA responses.
In the current experiments, using automated assessments of struggling, we were able to quantify these behaviors and found replicable effects of prior stress on restraint-induced struggling. We also observed similarities between these behaviors and HPA activity after 30 minutes of restraint. We analyzed behavior during acute restraint after exposure to repeated restraint (Experiment 1 and Experiment 3) or repeated swim (Experiment 2 and Experiment 4). In Experiment 1 we hypothesized that repeated restraint would lead to decreases in struggling during the 5th restraint, in parallel to HPA habituation seen with repeated restraint [12]. In Experiment 2 we hypothesized that prior exposure to repeated swim would lead to increases in struggling during heterotypic restraint, in similarly to HPA facilitation seen in this paradigm [19]. In Experiment 3 and Experiment 4, we examined the role of glucocorticoids in modulating behavioral habituation to repeated restraint (Experiment 3) and behavioral facilitation after repeated swim (Experiment 4) via adrenalectomy. Finally, we examined the effects of repeated restraint on behavior during forced swim (Experiment 5) to determine whether the increased struggling seen in facilitated responses to novel restraint is reflective of a general increase in activity upon exposure to a heterotypic stress. Overall, our findings indicate that struggling is a reproducible, reliable behavioral response which is measurable during restraint stress, follows a pattern similar to that seen in HPA activity, and is modified by prior stress history.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles Rivers) weighing between 225–250 g were individually housed in plastic tub cages with ad libitum access to food and water. The housing room was on a 12:12 l:d cycle with lights on at 0600h. Animals were given a 5–7 day acclimation period prior to the beginning of experimentation or surgery and were briefly handled during this period. All stress and experimentation took place between 0800 – 1200h. All procedures were approved by the IACUC at the Children’s Hospital of Philadelphia.
Stress paradigms
Restraint
Animals were placed in open-ended Plexiglas cylindrical restrainers measuring 6.7 cm in diameter and 22.3 cm in length and placed in a clean cage with bedding which held the restrainer in place. Restraint lasted for 30 minutes/day, at which point animals were returned to their home cage. Immediately after the last restraint exposure (day 5 or day 8, depending on the experiment) animals were decapitated and trunk blood collected for ACTH and corticosterone analysis.
Forced swim
Acute and repeated forced swim animals were placed in a glass chromatography jar (18” high × 8.75” outer diameter, Fisher Scientific, St. Louis MO) filled two-thirds full of water measuring approximately 25°C. Rats were swum for 15min/day, a length of time allowing some comparability to the effects of 30 min stress while also being short enough for daily exposure to be tolerated. Animals given a single, acute forced swim exposure (Experiment 5) were decapitated immediately after swim and trunk blood was collected for analysis of ACTH and corticosterone.
Experimental Design
Experiment 1: Acute restraint vs. repeated restraint
We hypothesized that the amount of struggling elicited by restraint would habituate over 5 days of repeated restraint. Rats were divided into two groups: the repeatedly restrained group was restrained for 30 min/day for 5 days, while the acute restraint group was undisturbed until day 5, at which point they were restrained as well. Video of 30 min restraint was obtained on day 5.
Experiment 2: Acute restraint only vs. acute restraint following repeated swim
In contrast to Experiment 1, we hypothesized that we would see facilitation in struggling during novel, heterotypic restraint on day 5 after 4 days of repeated forced swim compared to acute restraint alone. Rats were again divided into two groups: the repeated swim group was placed in a swim tank for 15min/day for 4 days, while the acute group was undisturbed. On d5 all animals were videotaped during 30 min restraint.
Experiment 3: Effects of adrenalectomy (ADX) on behavior during acute restraint vs. repeated restraint
Animals were either ADX or sham operated, as described below. After recovery, all animals were restrained for 8 days, during which video was captured on day 1 (acute response), and days 5 and 8 (habituated responses). We studied both day 5 and day 8 to allow for comparisons to our previous studies [11,12].
Experiment 4: Effects of ADX on behavior during acute restraint vs. acute restraint following repeated swim
Animals were divided into a 2×2 design: ADX vs. sham, and repeated swim vs. no stress. After recovery from surgery, on days 1–4 all repeated swim animals were placed in a Porsolt tank for 15 min/day, while acute animals were undisturbed. On day 5 all animals were videotaped during 30 min restraint.
Experiment 5: Effects of repeated restraint on behavior during forced swim
It is possible that the increases in struggling seen during exposure to novel restraint after repeated swim in Experiment 2 and 4 reflect general increases in movement after repeated stress exposure. If this were so, one might expect an increase in movement during any novel stress after repeated exposure to a homotypic stress. We tested this hypothesis using two groups of animals: one group was repeatedly restrained for 7 days, while the other remained in the home cage. On day 8 all animals were videotaped during 15 min of heterotypic forced swim.
Adrenalectomy
In Experiment 3 and Experiment 4, which examined the effect of glucocorticoids on struggling behavior, all animals underwent bilateral surgical removal of the adrenals (ADX) or a sham surgery (the adrenals were exposed but not removed). ADX animals received 100mg 35% corticosterone pellets subcutaneously, which provided a steady low dose of corticosterone at approximately the average daily value for intact rats [1]. Sham operated animals received 100mg pellets of cholesterol. ADX animals were also given 0.5% saline to drink for the duration of the experiment to prevent alterations in sodium balance that result from loss of adrenal hormones [21]. After surgery, animals were given between 5–7 days recovery before beginning experimentation. Completeness of adrenalectomies was verified by radioimmunoassay for corticosterone.
Video acquisition
Behavior during restraint
On test day video acquisition began by recording a background image that included the restrainers to subtract out of the final analysis. The camera used for acquisition (Panasonic WV-BP334), connected to an IBM ThinkCentre computer, was positioned and focused such that the restrainers were filmed from above and occupied as much of the screen width as possible. Videos were acquired directly onto the computer hard drive as black/white MPEG-2 files with MediaCruise encoding software (Canopus, San Jose, CA).
Behavior during forced swim
As with restraint, video acquisition began by recording the filled tanks in position to obtain a background image. The camera was positioned such that four tanks, positioned side by side and filmed from the side, took up approximately 90% of the screen width. Videos were acquired as described above.
Behavioral analysis
Automatic coding of behavior was analyzed using the EthoVision Pro 3.1 video analysis software (Noldus Information Technology, Leesburg, VA) using the mobility parameter for analysis of behavior during both restraint and forced swim. Briefly, the software is able to give an index of an animal’s mobility by detecting the extent of the animal as a field of pixels, and then assessing the percent pixel change between samples of the video. For all automated analysis the subtraction method of detection was used, detecting all objects different from background.
Automated analysis of restraint
Detection thresholds were set as to ensure that the head and body of the animals were included in observation but the tail was excluded. Thresholds of percent pixel change were set prior to any automated analysis of restraint. These thresholds were based on our preliminary observation of 5 restrained animals by an observer experienced in assessing behavior. Based on our observations, parameters were set in EthoVision. to define three different levels of mobility: immobility, mobility (which we label as “light mobility” here to avoid confusion), and strong mobility. “Immobility” was visually indicated by an almost total lack of movement except for breathing was set to register between 0–2% pixel change. “Light mobility” was defined by smaller or slower movements of the head occurring throughout the 30 minute restraint, including both sniffing and most bouts of grooming, corresponding to between 2–6% pixel change. The “strong mobility” parameter was defined by the largest pixel change percentages (greater than 6%, and generally not higher than 15%), which occurred during various struggling/escape behaviors such as chewing on the restrainer, attempts to nose out, back out, or turn around in the restrainer, and rotation within the restrainer. The sampling rate was 5 times/second, allowing for fine-tuned distinctions of mobility. All of these parameters were set based on preliminary observations and before any experimentation was conducted. Data were analyzed both as 30 minute totals and (in the case of strong mobility) in 5-minute bins.
Correlation of manual coding with automated coding of behavior during restraint
As struggling behavior during restraint as not been previously been well quantified, we assessed whether the strong mobility measurements obtained by analysis in EthoVision corresponded to observers’ estimations of struggling behavior. For Experiment 1, two coders blind to experimental condition of the rats and familiar with the kinds of movements associated with struggling (chewing on the restrainer, attempts to nose out, back out, or turn around in the restrainer, and rotation within the restrainer) measured the total time spent struggling over the 30 minute restraint for each animal in this experiment. Overall, these coders scores were very highly correlated with each other (r (13) = .78, p ≤ 0.005) and the average of their scores were very highly correlated with the total time spent strongly mobile as assessed with EthoVision (r (13) = .72, p ≤ 0.01), indicating that “strong mobility” is very closely associated with struggling as assessed by human observers. Furthermore, an unpaired t-test revealed significant differences between the acutely and repeatedly restrained rats in total time spent struggling over the 30 minute restraint as assessed by human coders (acute restraint mean = 95.6 sec, S.E.M. = 24.6; repeated restraint mean= 36.9 sec, S.E.M.= 12.8; t(13) = −2.193, p ≤ 0.05), paralleling the results seen in the automatic coding of strong mobility (see Results – Experiment 1).
Automated analysis of forced swim
The three mobility parameters (immobility, light mobility, strong mobility) were set to follow as closely as possible the distinctions commonly used to describe behavior during forced swim, as described below. To validate this method of measuring behavior during forced swim, a set of 8 naive animals separate from the experiments described here were each swum for 5 minutes. During this time, video was simultaneously obtained of the side view (as in the current set of studies) and of the top-down view (as forced swim behavior is typically coded). Both the top-down and side views were hand-coded by an observer expert at coding forced swim behavior [23]. These scores were converted to percent time spent immobile, swimming, or climbing/diving for comparison to the percent time spent immobile, lightly mobile, and strongly mobile, respectively, obtained by adjusting the percent pixel changes and sampling rate in EthoVision. Using these parameters immobility was indicated by less than 18.7% pixel change corresponding with a lack of movement other than that needed to keep the head afloat. Light mobility/swimming was indicated by 18.8–22.3% pixel change and corresponded to movements of the limbs associated with swimming/less severe than those associated with climbing. Strong mobility/climbing was indicated by greater than 22.3% pixel change and corresponded to large movements associated with attempts to escape the swim tank, including vigorous climbing near the sides of the tank and diving to the bottom of the tank. For swim videos, the sampling rate was averaged over 25 samples (1 averaged mobility score per 5 seconds), which minimized the influence of small variations of movement in the automatic analysis and produced scores similar to those obtained by hand coding. In addition to acquiring mobility data for analysis of experiment 5, we also acquired and analyzed the distance moved within the tank via center-of-mass tracking, as this has been previously used as an inverse measure of immobility in the FST [13] and could be used to confirm results of the mobility analysis. All data were analyzed as 15 minute totals and immobility, light mobility, and strong mobility were also analyzed in 5 minute bins.
Hormone assays
At the end of 30 min restraint or 15 min swim, trunk blood was collected on ice into 15 ml conical tubes containing 100 µl sodium EDTA to prevent coagulation. Whole blood was centrifuged at 2500 rpm for 15 min. The plasma was reserved and frozen at − 20°C. Plasma ACTH and corticosterone were measured using kits from MP Biomedicals (Orangeburg, NY). The minimum levels of detection for ACTH and corticosterone were 5.7 pg/ml and 0.6 µg/dl respectively. Intra- and interassay variability was less than 10%.
Statistical Analyses
Omnibus analyses of struggling behavior and hormones
All automated data, hand-coded behavioral data, and 30 min ACTH and corticosterone concentrations were analyzed with Statview software. For Experiment 1, Experiment 2, and Experiment 5, unpaired t-tests were conducted on total immobility, mobility, strong mobility or hormone concentrations, and in experiment 5, total distance traveled was also analyzed. In Experiment 3 repeated measures ANOVA [Surgery (sham, ADX) × Day (one, five, eight)] was conducted on total immobility, mobility, and strong mobility, and an unpaired t-test was used to compare hormone levels. In Experiment 4, 2×2 ANOVAs [Surgery (sham, ADX) × Stress (acute restraint, repeated swim followed by acute restraint)] were conducted on total immobility, mobility, strong mobility and hormone concentrations.
Timecourse analyses of struggling behavior
For Experiment 1–Experiment 4, repeated measures ANOVAs were conducted on strong mobility measurements across 30 minute restraint divided into 5 minute timepoints, with timepoint as the repeated measure. For Experiment 1, this analysis was Stress (acute restraint, repeated restraint) × Timepoint (0–5 min, 5–10, 10–15, 15–20, 20–25, 25–30); Experiment 2, Stress (acute restraint, repeated swim followed by acute restraint) × Timepoint; Experiment 3, Surgery (sham, ADX) × Day of restraint (one, five, eight) × Timepoint; Experiment 4, Surgery × Stress (acute restraint, repeated swim followed by acute restraint) × Timepoint. For Experiment 5 repeated measures ANOVA was conducted on measurements of all swimming behaviors (immobility, light mobility/swimming, and strong mobility/climbing) divided into 5 minute timepoints, with timepoint as the repeated measure, making the analysis Stress (acute swim, repeated restraint followed by swim) × Timepoint (0–5 min, 5–10, 10–15). All significant effects were followed by Fisher's post hoc tests. The significance levels for all tests were set to p ≤ 0.05.
Results
Experiment 1: Acute Restraint vs. Repeated Restraint
Behavioral analyses
These data are presented in Figure 1. We found a significant difference between acutely and repeatedly restrained rats in strong mobility over the 30 minute restraint (t (13) = −2.1, p ≤ 0.05). Acutely restrained rats spent more time strongly mobile than repeatedly restrained rats (Fig 1a). Unpaired t-tests revealed no significant differences between acutely and repeatedly restrained rats in total time spent either immobile or lightly mobile (engaging in small movements not corresponding to struggling) during restraint on day 5.
Figure 1.
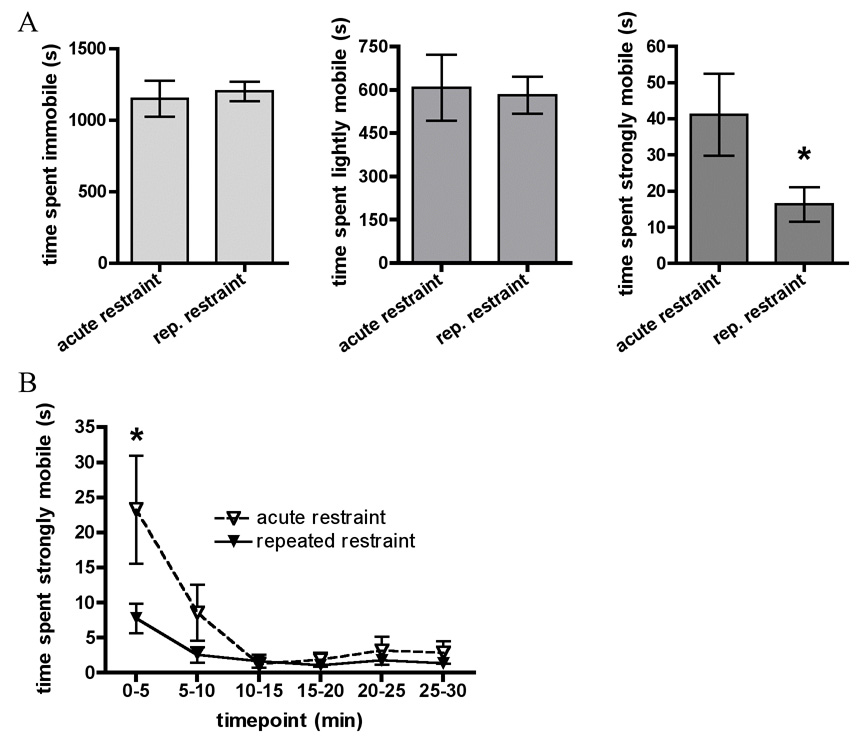
In Experiment 1, animals were restrained on day 5 with (repeated restraint group) or without (acute restraint group) 4 prior days of repeated restraint stress. A. Graphs show total time spent immobile, lightly mobile, and strongly mobile (struggling) during 30 minute restraint on day 5. B. Timecourse of time spent strongly mobile (struggling) across 30 minute restraint on day 5. All data are expressed as mean ± SEM. Asterisks indicate repeated restraint group values significantly different from acute restraint group values.
We then analyzed time spent strongly mobile in 5 minute increments to examine changes in strong mobility at different timepoints within the 30 minute restraint period. Repeated measures ANOVA on Stress × Timepoint, with Timepoint as the repeated measure, revealed a significant Main effect of Stress (F (1,13) = 4.4, p ≤ 0.05), a significant Main effect of Timepoint (F (5, 65) = 10.7, p ≤ 0.001) and a significant Interaction effect (F (5, 65) = 3.2, p ≤ 0.01). The significant Main effect of Stress indicated that repeatedly restrained rats showed lower levels of struggling overall than the acutely restrained rats. The significant main effect of time indicated that in all animals, struggling was highest during the first 5 minutes (0–5 min) of restraint than at any other time. Fisher’s post hoc analyses of the significant interaction test revealed that acutely restrained animals spent significantly more time strongly mobile during the first 5 minutes than repeatedly restrained rats at all timepoints and acutely stressed rats at any other timepoints.
HPA response
ACTH concentrations at the end of 30min restraint were significantly reduced in repeatedly restrained rats as compared to acutely restrained rats (t (13) = −2.1, p ≤ 0.05; Table 1). Corticosterone levels at 30 minutes were not different between groups at this timepoint.
Table 1.
Plasma ACTH and corticosterone levels collected at the end of 30 minute restraint on day 5 (Experiment 1, Experiment 2, and Experiment 4) or day 8 (Experiment 3). In experiment 1 and experiment 2, animals were acutely restrained on day 5 with or without 4 days prior repeated restraint (Experiment 1) or repeated swim (Experiment 2). In Experiment 3 and Experiment 4, animals were either sham operated or adrenalectomized (ADX) prior to repeated stress, and blood samples were taken at the end of restraint on day 8 or 5 to confirm ADX. All data are expressed as mean ± SEM.
| Condition | ACTH | Corticosterone | |
|---|---|---|---|
| Experiment 1 | acute restraint | 177.0 ± 48.4 | 19.1 ± 5.0 |
| Repeated restraint | 70.6 ± 21.9 * | 21.7 ± 9.3 | |
| Experiment 2 | acute restraint | 194.5 ± 23.7 | 25.5 ± 2.9 |
| Repeated swim + acute restraint | 209.2 ± 32.7 | 23.5 ± 2.2 | |
| Experiment 3 | Sham + repeated restraint | 98.8 ± 17.8 | 16.4 ± 2.9 |
| ADX + repeated restraint | 1594.5 ± 87.1 † | 3.9 ± 0.4 † | |
| Experiment 4 | Sham + acute restraint | 201.1 ± 99.5 | 14.7 ± 2.1 |
| Sham + repeated swim + acute restraint | 211.3 ± 49.7 | 26.4 ± 3.0 * | |
| ADX + acute restraint | 1086.9 ± 134.6 † | 2.4 ± 0.3 † | |
| ADX + repeated swim + acute restraint | 1383.5 ± 85.8 † | 2.2 ± 0.3 † |
Asterisks indicate repeated stress group values are significantly different than comparable acute restraint group values.
Crosses indicate ADX values are significantly different than comparable sham group values.
Experiment 2: Acute Restraint With or Without Prior Repeated Forced Swim Exposure
Behavioral responses
These data are presented in Figure 2. Repeatedly swum animals spent significantly more total time strongly mobile during novel restraint than acutely restrained rats, (t(20) = −2.6, p ≤ 0.01). No differences were seen between repeatedly swum animals in novel restraint and acutely restrained rats in total time spent immobile or lightly mobile during restraint.
Figure 2.
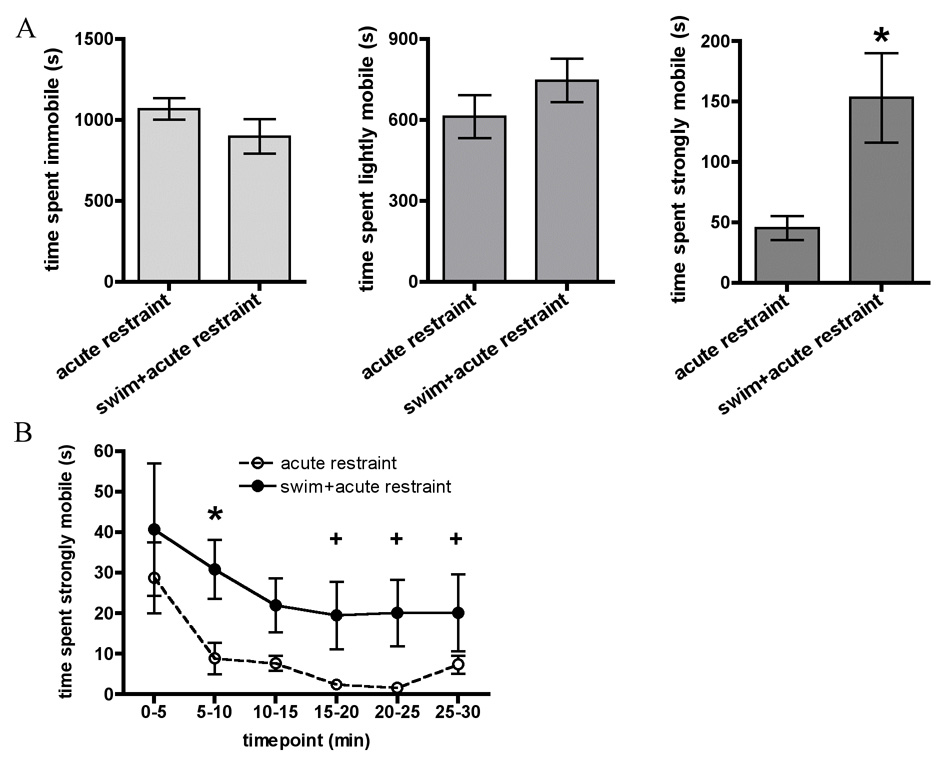
In Experiment 2, animals were restrained on day 5 after 4 days of repeated forced swim (swim + acute restraint group) or without prior swim (acute restraint group). A. Graphs show total time spent immobile, lightly mobile, and strongly mobile (struggling) during 30 minute restraint on day 5 in animals. B. Timecourse of time spent strongly mobile (struggling) across 30 minute restraint on day 5. All data are expressed as mean ± SEM. Asterisk indicates that acutely restrained rats exhibited higher strong mobility than acutely restrained rats after swim throughout the 30min period of testing.
Repeated measures ANOVA on Stress × Timepoint revealed a Main effect of Stress (F(1, 20) = 7.0, p ≤ 0.01) and a Main effect of Timepoint (F(5, 100) = 4.2, p ≤ 0.001) but no Interaction effect. The Main effect of Stress indicated that overall, repeatedly swum animals spent more time strongly mobile during novel restraint than acutely restrained animals. Fisher’s post-hoc analyses of the significant Main effect of Timepoint indicated that strong mobility in the first five minutes was significantly higher than at any other time points (independent of acute or repeated stressed groups).
HPA responses
ACTH and corticosterone levels at the end of 30 minute restraint were similar between animals that were experiencing acute restraint and animals that had previously experienced repeated swim (Table 1).
Experiment 3: Acute vs. Repeated Restraint in Adrenalectomized Animals
Behavioral Responses
Repeated measures ANOVAs (Surgery × Day) were conducted for total time spent immobile, lightly mobile, and strongly mobile (Figure 3a). Importantly, total time spent strongly mobile showed no effect of Surgery, but a significant Main effect of Day (F (2, 32) = 10.5, p ≤ 0.001) showing in all animals a significant decrease in strong mobility between days one and five, and between days one and eight, and no difference between days five and eight. In immobility, there was a significant Main effect of Day (F (2, 32) = 4.9, p ≤ 0.01), such that total immobility significantly increased between days one and five, but no difference was observed between days five and eight or between days one and eight. Changes in light mobility between days showed a similar pattern, revealing a significant Main effect of Day (F (2, 32) = 4.2, p ≤ 0.05) showing a significant decrease in time spent lightly mobile between days one and five, and no difference between days five and eight or days one and eight. No effect of Surgery was found in any analysis.
Figure 3.
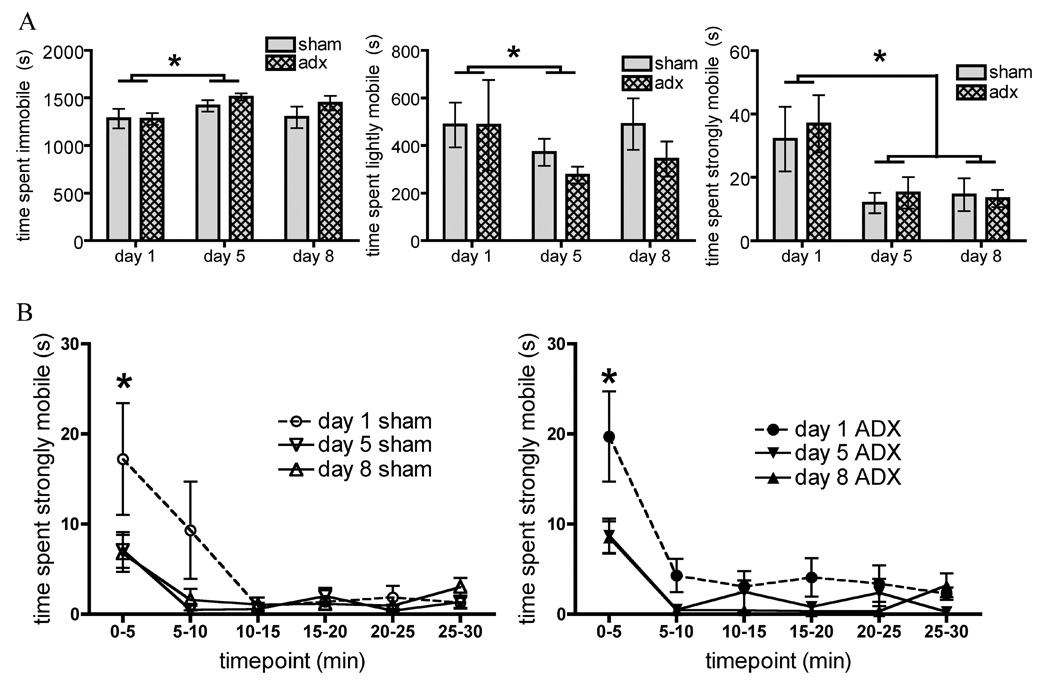
In Experiment 3 animals were first sham operated (sham) or adrenalectomized (ADX) and replaced with subcutaneous corticosterone pellets prior to 8 days repeated restraint. A. Graphs show total time spent immobile, lightly mobile, and strongly mobile (struggling) in both groups on days 1 (acute restraint), 5, and 8 of restraint. B. Timecourse of time spent strongly mobile (struggling) across 30 minute restraint on days 1, 5, and 8, divided by surgery. All data are expressed as mean ± SEM. Asterisks indicate repeated restraint group values significantly different from overall acute restraint group values.
Repeated measures ANOVA (Surgery × Day of restraint × Timepoint) on time spent strongly mobile each day divided into 5 minute increments revealed a significant Main effect of Day (F(2, 32) = 10.5, p ≤ 0.001) indicating that strong mobility was significantly decreased from day one to day five, and day one to day 8, with no difference between days five and eight. A significant Main effect of Timepoint (F(5, 80) = 18.2, p ≤ 0.001) was also seen, indicating that strong mobility was greater during the first five minutes of restraint than any other time period, regardless of the day or surgical treatment. Finally, a significant Day × Timepoint Interaction (F(10, 160) = 5.4, p = 0.001) was seen. Post-hoc analyses indicated that both sham and ADX animals showed greater levels of strong mobility in the first 5 minutes on day one than on days five and eight. There were no other significant effects.
HPA Responses
Blood plasma samples were taken at the end of stress on day 8 to confirm ADX (table 1). Unpaired t-tests between sham operated and ADX animals after 8 days repeated restraint showed a significant difference in ACTH (t(16) = −13.4, p = 0.001) and corticosterone levels (t(16) = 5.3, p ≤ 0.001) between groups. ADX animals showed significantly increased ACTH concentration, consistent with a lack of corticosterone negative feedback, and significantly lower corticosterone levels consistent with corticosterone replacement via the subcutaneous pellets, compared to sham-adrenalectomized animals.
Experiment 4. Acute Restraint With or Without Prior Swim Exposure in Adrenalectomized Animals
Behavioral Responses
These results are shown in Figure 4. A 2×2 ANOVA conducted on total time spent strongly mobile on day 5 showed a significant Main effect of Stress (F (1, 27) = 6.5, p ≤ 0.01), indicating that animals exposed to repeated forced swim prior to restraint on day 5 spent more total time strongly mobile than acutely restrained animals. 2×2 ANOVA (Surgery × Stress) conducted on total time spent immobile or lightly mobile during restraint on d5 showed no significant effects. No effect of Surgery and no Interaction effect were observed in any analysis.
Figure 4.
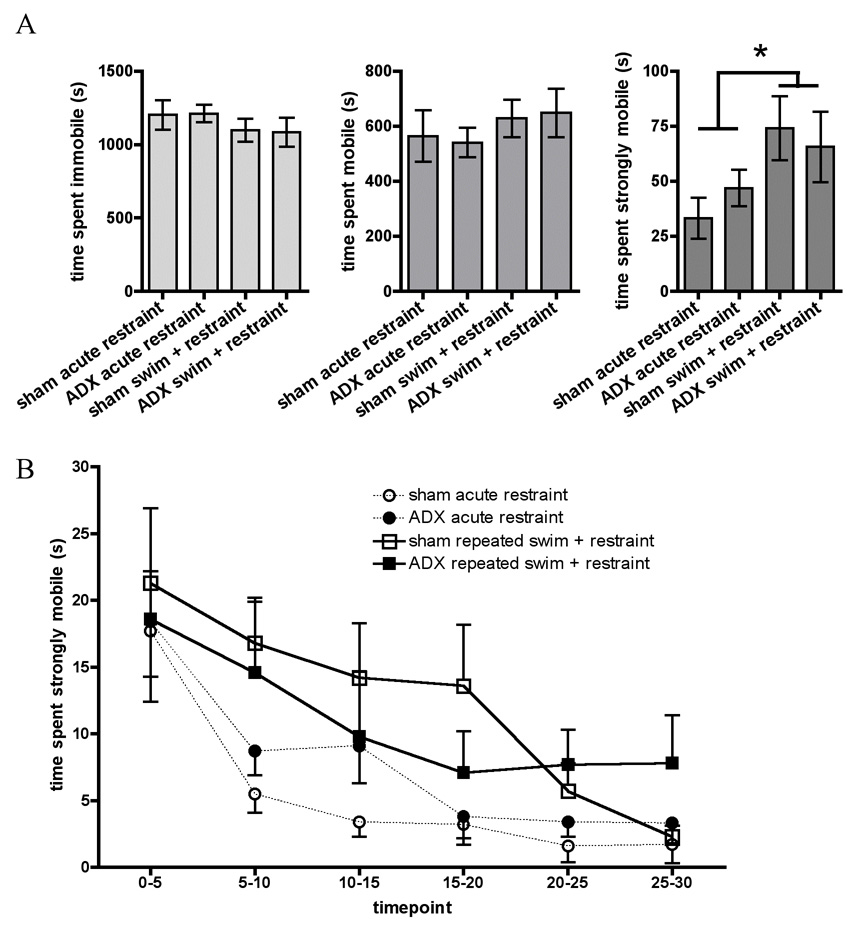
In Experiment 4 animals were first sham operated (sham) or adrenalectomized (ADX) and replaced with subcutaneous corticosterone pellets, then were either undisturbed until restraint on day 5 (acute restraint) or given 4 days of repeated forced swim prior to restraint on day 5 (swim + acute restraint). A. Graphs show total time spent immobile, lightly mobile, and strongly mobile (struggling) in sham and ADX animals during 30 minute restraint on day 5. B. Timecourse of time spent strongly mobile (struggling) across 30 minute restraint on day 5, divided by both stress history and by surgery. All data are expressed as mean ± SEM. Asterisks indicate overall repeated swim + restraint group values significantly different from overall acute restraint values.
Repeated measures ANOVA (Surgery × Stress × Timepoint) were conducted on time spent strongly mobile on day 5 divided into 5 minute timepoints. A Main effect of Stress (F (1, 28) = 5.8, p ≤ 0.05), indicated that, overall, animals which received repeated swim prior to restraint showed significantly elevated levels of strong mobility as compared to acutely restrained animals, regardless of surgery (comparison not specifically shown). A Main effect of Timepoint during restraint (F (5, 140) = 16.3, p ≤ 0.001) was also seen, indicating that strong mobility was higher during the first 5 minutes than at all other timepoints, higher at 5–10 minutes than at any time in the last 15 minutes of restraint, and higher at 10–15 minutes than at any time in the last 10 minutes of restraint. No effects of Surgery and no significant Interactions were seen. Therefore, animals exposed to repeated swim + restraint exhibited higher strong mobility over the 30 minute period of restraint compared to animals exposed to restraint alone. Adrenalectomy did not significantly alter this finding.
HPA Responses
A 2×2 ANOVA (Surgery × Stress) conducted on ACTH levels at the end of 30 min restraint on day 5 showed no Main effect of Stress, but a significant Main effect of Surgery (F (1, 27) = 97.9, p ≤= 0.001) indicating that the ACTH levels of ADX animals were significantly elevated, regardless of stress history. No Interaction effects were seen.
2×2 ANOVA (Surgery × Stress) on corticosterone levels at the end of 30 min restraint showed significant Main effects of Stress (F (1, 27) = 4.3, p ≤ 0.05), indicating that overall, plasma corticosterone concentrations were significantly higher in repeatedly swum animals in acute restraint than naïve animals in acute restraint. There was also a Main effect of Surgery (F (1, 27) = 90.5, p ≤ 0.001), indicating that the sham operated animals had significantly higher corticosterone levels than ADX animals overall. A significant Interaction effect (F (1, 27) = 4.8, p ≤ 0.05) was also seen, indicating that increase in corticosterone due to prior swim stress was only significant in the sham operated group.
Experiment 5. Forced swim exposure in naïve vs. repeatedly restrained animals
Behavioral Analyses
These data are presented in Figure 5. No differences were seen in between animals receiving acute exposure to 15 minute swim, compared to animals receiving 15 minute swim after 7 previous days of 30 minute restraint, showed no differences in time spent immobile, lightly mobile (corresponding to swimming behavior), or strongly mobile (corresponding to climbing behavior) over the total 15 minutes (not shown).
Figure 5.
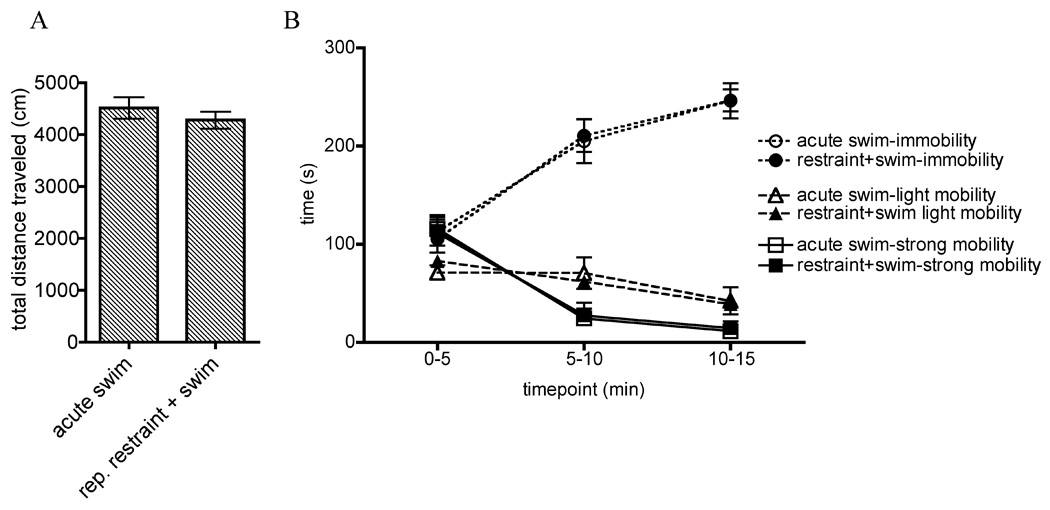
A. In Experiment 5, animals were placed in forced swim for 15 min with (rep. restraint + swim group) or without (acute swim group) 7 prior days of repeated restraint. A. Graphs show total distance traveled during 15 minute forced swim on day 8. B. Timecourse of times spent immobile, lightly mobile, and strongly mobile across 15 minute swim on day 8. All data are expressed as mean ± SEM.
Following the methods of Hedou and colleagues [13], we also examined total distance traveled via center of mass tracking, which was verified previously as an inverse measure of immobility in the FST. An unpaired t-test comparing total distance traveled in acutely swum rats versus rats exposed to repeated restraint prior to acute swim revealed no differences between groups. We examined the correlation between total distance traveled and total immobility, light mobility, and strong mobility and found a negative correlation between immobility and total distance traveled (r (19) =−.46, p ≤ 0.05) and a corresponding positive correlation between strong mobility and total distance traveled (r (19) = .48, p ≤ 0.05).
Repeated measures ANOVAs (Stress × Timepoint) were conducted on time spent immobile, mobile, and strongly mobile divided in 5 minute increments. A significant Main effect of Timepoint in all three analyses was observed, such that immobility increased over the 15 minute period in all animals (F (2, 36) = 120.1, p ≤ 0.001) and mobility/swimming and strong mobility/climbing decreased in all animals (F (2, 36) = 13.2, p ≤ 0.001 and F(2, 36) = 120.7, p ≤ 0.001, respectively). Post hoc analyses indicate that immobility significantly increased across all three timepoints, light mobility was significantly decreased at the 10–15 minute timepoint compared to the first and second 5 minutes, and strong mobility was significantly higher in the first 5 minutes than the remainder of the swim. No effects of Stress and no Interactions were seen.
Discussion
The experiments presented here indicate that struggling during restraint is a stress-induced behavior that is consistent and readily quantifiable. Struggling can be modified by prior stress history in a manner similar to the HPA response, but does not seem to be regulated by stress-induced increases in circulating glucocorticoids. In Experiment 1, we found that in comparison with acute restraint, repeated exposure to restraint significantly reduced the amount of restraint-elicited struggling. Likewise, in Experiment 2, animals that were repeatedly swum struggled significantly more during acute restraint than naïve animals in acute restraint, demonstrating behavioral facilitation (Figure 2). In both of these experiments, acutely restrained animals displayed a stereotypical struggling response to restraint, with levels of struggling highest during the first 5 minutes of restraint and showing rapid within-restraint habituation. Thus the reduction of struggling in the habituated animals in Experiment 1 is most clearly visible during the first 5 minutes of restraint, after which point all animals largely stopped struggling. In contrast, the facilitation of struggling in Experiment 2 is significant only after the first 5 minutes of restraint, when the facilitated animals continue to struggle at a point at which acutely restrained animals no longer struggle.
For the most part, HPA responses followed what was expected in terms of habituation to homotypic stress and facilitation to heterotypic stress exposure, a pattern also observed in the behavioral struggling responses. The behavioral habituation in Experiment 1 was associated with habituation of ACTH though corticosterone did not habituate (Table 1). The most likely reason is that we only collected one blood sample at 30 minute and it is possible that corticosterone habituates at a time following termination of restraint. The possibility remains that changes in adrenal responsivity or intra-adrenal mechanisms prevent habituation at the adrenal level though many studies have observed habituation of both ACTH and corticosterone [4,10,11,16]. In animals exposed to restraint after repeated swim in Experiment 2, ACTH and corticosterone levels are similar to those of acutely restrained rats. Therefore, repeated swim rats exhibit facilitated HPA responses to novel since their HPA responses match those of acutely stressed rats, as defined by Dallman and Jones [8]. We have previously observed facilitation of HPA responses to restraint after repeated swim [19].
As discussed in the introduction, struggling in rats during acute immobilization has been measured in a few studies, but this is the first detailed description of the different behaviors that we collectively classify as “struggling.” These behaviors include chewing on the restrainer, attempts to nose out, back out, or turn around in the restrainer, and rotation within the restrainer. The use of automated analysis software in our experiments greatly streamlined our analysis of struggling behavior in restrained rats, t. Nevertheless, the software is unable to note distinctions between the different sorts of large movements that might trigger “strong mobility” / struggling. Therefore, we do not know whether in a given experiment one group spent more time chewing, or another spent more time turning around and these behaviors could reflect very different states of the animal. Future investigation of struggling behavior may find analysis of these individual behaviors informative.
A number of behavioral tasks currently exist which can be used to assess anxiety-like or depressive-like behaviors in an animal. However, the experiments presented here do not address whether one or more psychological states are reflected by increased or decreased struggling behavior. It has been previously shown that levels of struggling during immobilization are decreased by peripheral administration of GABA agonists [27] and morphine [26], and increased by naloxone [26]. Based on these data, it is possible that struggling may reflect an anxiety-like state. If a relationship between this behavior and an anxiety or depressive-like state is found, struggling could be used to provide a measure of an animal's behavioral state while permitting simultaneous analysis of peripheral or central physiological markers in response to uninterrupted restraint. This potential is especially pertinent to designs involving habituation to repeated restraint or facilitation to novel restraint. For instance, while in these studies we did not wish to jeopardize novel behavioral data to obtain multiple blood samples, our results indicate that significant differences in struggling are observed within the first 15 minutes of restraint. Subsequent experiments using this measure could potentially obtain blood samples repeatedly after the first 15 minutes of restraint, allowing more direct comparisons of behavioral measures with neural measures such as HPA activity and mRNA expression in specific stress-regulatory brain regions.
Given the similarities between struggling behavior and HPA activity seen in Experiment 1 and Experiment 2, it was possible that struggling behavior is regulated by stress-induced increases in circulating glucocorticoids. In Experiment 3 and Experiment 4, we examined the effects of eliminating the corticosterone response to stress on the generation of acute, habituated, or facilitated struggling responses to restraint. Adrenalectomy with corticosterone replacement prior to the beginning of repeated restraint or swim had no statistically significant effects on the magnitude of struggling in acutely restrained animals, or on the development of behavioral habituation (Figure 3) or facilitation (Figure 4). We conclude that stress-induced increases in glucocorticoids do not regulate struggling to restraint. However, additional study may be required before we can be sure of this interpretation. In Experiment 3, the struggling response habituated over repeated restraint, over the course of which corticosterone release would progressively diminish even in sham operated animals. In contrast, in Experiment 4 struggling was expected to increase in animals exposed to restraint after repeated swim. While the statistical analyses did not reveal significant effects of ADX on behavioral facilitation, the graphs appear to indicate that facilitation in struggling was somewhat blunted in ADX animals restrained after repeated swim compared to sham animals. It is possible that in this experiment, the corticosterone replacement was insufficient to allow behavioral facilitation to the same level as the sham operated animals. A future study could test this idea by examining behavioral facilitation in ADX animals with varying levels of corticosterone replacement. No corticosterone replacement may abolish behavioral facilitation, while higher levels may be required for behavioral facilitation to proceed normally. At present, however, our results indicate that 1) struggling during restraint is influenced by stress history, but 2) this modulation does not appear to be regulated by stress-induced increases in glucocorticoids.
The results of Experiment 2 and Experiment 4, showing an increase in struggling to restraint after repeated swim, raise the question of whether simple exposure to any heterotypic stressor after a period of repeated homotypic stress increases movement in general, or whether the facilitation of struggling seen here is specific to restraint. In Experiment 5 we tested this hypothesis by examining behavior during forced swim with or without 7 days of previous repeated restraint, and found no effect on immobility, light mobility, or strong mobility (mapping onto immobility, swimming, and climbing, respectively) during forced swim (Figure 5). We also analyzed total distance traveled, which is a previously validated index of forced swim behavior [13] and also found no difference between groups. However, total distance traveled was found to be significantly negatively correlated with immobility and significantly positively correlated with strong mobility, a finding similar to previous work using total distance traveled [13]. Overall, these results do not support the idea that movement is increased generally in response to a heterotypic stressor after a period of homotypic stress. There are of course several important caveats to this interpretation of the above experiment. First, repeated restraint is not as severe a stressor as repeated forced swim [9,23]. It is possible that using a stronger repeated stressor might have elicited differences in overall mobility during swim in Experiment 5. Second, the test swim in Experiment 5 may have required more movement in general than the test restraints in Experiment 2 and Experiment 4. Animals cannot remain truly immobile during forced swim in the same way as is possible during restraint, and as a result movement during swim might be high enough in general to obscure group differences. Regardless, these caveats do not contradict the idea that struggling during restraint is a behavior distinct from the behaviors seen during forced swim and does not reflect a non-specific increase in motor activity after repeated stress.
It is possible that struggling during restraint is stimulated by some of the same circuitry that stimulates the HPA axis, which may account for the similarity between the behavioral and hormonal responses. While the present studies do not address what brain areas may be involved in struggling during acute restraint or the habituation or facilitation of this behavior, other literature has shown an interesting relationship between amygdala activity and struggling during acute immobilization. Strain differences in amygdala excitability, which result in either high or low propensity towards seizure kindling, lead respectively to high or low HPA activity and struggling during acute immobilization [2,17,18]. Additionally, induction of dentate gyrus LTP via basolateral amygdala stimulation leads to decreases in struggling during acute immobilization [14]. It is possible that the co-regulation of HPA and behavioral responses may be related to the functioning of one or more amygdalar nuclei and potentially other limbic structures, but this remains a question to be addressed by future studies.
The studies presented here demonstrate that struggling during restraint can be bidirectionally modified by prior stress history, habituating and facilitating in parallel with HPA activity in response to restraint. There are likely a number of significant relationships that remain to be found between struggling and other physiological and neural changes induced by restraint. There is already a literature indicating that greater amounts of struggling during immobilization is associated with altered immune system functioning [2], increased severity of gastric ulcers [14,27], increased lactate levels in blood [22] and lactic acid in muscle indicative of hyperglycemia and metabolic acidosis [7]. In these studies the amount of struggling observed was directly related to the severity of negative stress-induced physiological outcomes. These physiological changes, induced by acute or repeated stress and associated with struggling, may habituate and facilitate along with struggling, making it a potentially important and useful behavioral index of coping in response to restraint.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z. Influence of psychogenic and neurogenic stressors on endocrine and immune activity: differential effects in fast and slow seizing rat strains. 1997;11:63–74. doi: 10.1006/brbi.1997.0482. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. 2000;20:5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 7.Bush M, Custer R, Smeller J, Bush LM. Physiologic measures of nonhuman primates during physical restraint and chemical immobilization. 1977;171:866–869. [PubMed] [Google Scholar]
- 8.Dallman MF, Jones MT. Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone ssecretion on subsequent stress responses in the rat. Endocrinology. 1973;92:1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- 9.Dal-Zotto S, Marti O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav.Brain Res. 2000;114:175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 10.Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. 2007;51:95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Grissom N, Kerr W, Bhatnagar S. Noradrenergic receptor activity in the basolateral amygdala (BLA) modulates adaptation to repeated stress, Program No. 198.13. 2007 [Google Scholar]
- 13.Hedou G, Pryce C, Di Iorio L, Heidbreder CA, Feldon J. An automated analysis of rat behavior in the forced swim test. 2001;70:65–76. doi: 10.1016/s0091-3057(01)00575-5. [DOI] [PubMed] [Google Scholar]
- 14.Henke PG. Potentiation of inputs from the posterolateral amygdala to the dentate gyrus and resistance to stress ulcers formation in rats. 1990;48:659–664. doi: 10.1016/0031-9384(90)90207-k. [DOI] [PubMed] [Google Scholar]
- 15.Himmelsbach CK, Gerlach GH, Stanton EJ. A method for testing addiction, tolerance, and abstinence in the rat. J. Pharmacol. Exp. Ther. 1935;53:179–188. [Google Scholar]
- 16.Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre DC, Kent P, Hayley S, Merali Z, Anisman H. Influence of psychogenic and neurogenic stressors on neuroendocrine and central monoamine activity in fast and slow kindling rats. 1999;840:65–74. doi: 10.1016/s0006-8993(99)01771-0. [DOI] [PubMed] [Google Scholar]
- 18.Merali Z, Kent P, Michaud D, McIntyre D, Anisman H. Differential impact of predator or immobilization stressors on central corticotropin-releasing hormone and bombesin-like peptides in Fast and Slow seizing rat. 2001;906:60–73. doi: 10.1016/s0006-8993(01)02556-2. [DOI] [PubMed] [Google Scholar]
- 19.Mercer B, Grissom N, Bhatnagar S. Repeated swim enhances hypothalamic-pituitary-adrenal (HPA) responses to subsequent homotypic or heterotypic stressors. Soc. Neurosci. Ab. 2006 [Google Scholar]
- 20.Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohara M, Cadnapaphornchai MA, Summer SN, Falk S, Yang J, Togawa T, Schrier RW. Effect of mineralocorticoid deficiency on ion and urea transporters and aquaporin water channels in the rat. 2002;299:285–290. doi: 10.1016/s0006-291x(02)02634-7. [DOI] [PubMed] [Google Scholar]
- 22.Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. 2002;16:123–132. doi: 10.1892/0891-6640(2002)016<0123:ashici>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 24.Smriga M, Torii K. L-Lysine acts like a partial serotonin receptor 4 antagonist and inhibits serotonin-mediated intestinal pathologies and anxiety in rats. 2003;100:15370–15375. doi: 10.1073/pnas.2436556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanton EJ. Dihydromorphinone hydrochloride (dilaudid): Its tranquilizing potency, respiratory depressant effects and addiction liability, as tested on the rat. J. Pharmacol. Exp. Ther. 1936;56:252–263. [Google Scholar]
- 26.Tanaka M, Kohno Y, Tsuda A, Nakagawa R, Ida Y, Iimori K, Hoaki Y, Nagasaki N. Differential effects of morphine on noradrenaline release in brain regions of stressed and non-stressed rats. 1983;275:105–115. doi: 10.1016/0006-8993(83)90422-5. [DOI] [PubMed] [Google Scholar]
- 27.Ushijima I, Mizuki Y, Hara T, Kudo R, Watanabe K, Yamada M. The role of adenosinergic, GABAergic and benzodiazepine systems in hyperemotionality and ulcer formation in stressed rats. 1986;89:472–476. doi: 10.1007/BF02412124. [DOI] [PubMed] [Google Scholar]


