Abstract
Acute promyelocytic leukemia (APML) most often is associated with the balanced reciprocal translocation t(15;17) (q22;q11.2) and the expression of both the PML-RARα and RARα-PML fusion cDNAs that are formed by this translocation. In this report, we investigated the biological role of a bcr-3 isoform of RARα-PML for the development of APML in a transgenic mouse model. Expression of RARα-PML alone in the early myeloid cells of transgenic mice did not alter myeloid development or cause APML, but its expression significantly increased the penetrance of APML in mice expressing a bcr-1 isoform of PML-RARα (15% of animals developed APML with PML-RARα alone vs. 57% with both transgenes, P < 0.001). The latency of APML development was not altered substantially by the expression of RARα-PML, suggesting that it does not behave as a classical “second hit” for development of the disease. Leukemias that arose from doubly transgenic mice were less mature than those from PML-RARα transgenic mice, but they both responded to all-trans retinoic acid in vitro. These findings suggest that PML-RARα drives the development of APML and defines its basic phenotype, whereas RARα-PML potentiates this phenotype via mechanisms that are not yet understood.
Acute promyelocytic leukemia (APML, or AML M3, based on the French-American-British classification system) comprises about 10% of all new cases of AML. This disease is characterized by the accumulation of promyelocytes in the marrow and the peripheral blood, and a predisposition for bleeding diatheses (reviewed in refs. 1 and 2). The most common genetic abnormality associated with APML is the balanced t(15;17) (q22;q11.2) reciprocal translocation that generates PML–retinoic acid receptor α (RARα) and RARα-PML fusion cDNAs (1). Although it is common to detect expression of both PML-RARα and RARα-PML mRNAs in primary APML cells, the direct link between PML-RARα expression and the development of APML was not demonstrated formally until transgenic expression of PML-RARα in early myeloid cells was shown to cause the development of APML in transgenic mouse models (3–5).
The first-generation mouse models demonstrated that expression of PML-RARα in early myeloid cells altered myeloid development in all mice and caused 15–20% of mice to develop a fatal APML-like disease after a latency of 6–18 months (3–5). These findings suggested that PML-RARα directly alters myeloid development and, in doing so, predisposes early myeloid cells to acquire additional mutations (second or subsequent hits) that ultimately cause transformation. The nature of these additional mutations is not yet known, although many patients with APML express the reciprocal RARα-PML cDNA, and a significant fraction have loss-of-function mutations in the p53 gene as well (6). The role of the reciprocal fusion for the development of APML has not been addressed previously in transgenic systems, probably because this fusion cDNA is small, containing only a few putative functional domains.
Expression analyses of patients with t(15;17) (q22; q11.2) APML disease have demonstrated that virtually all cases express the PML-RARα mRNA and that 70–80% express detectable amounts of RARα-PML mRNA (7–10). Although this observation (and the mouse models described above) suggested that RARα-PML is not required for development of APML, these studies did not rule out a role for the reciprocal cDNA as a potential second hit, which could affect the latency or penetrance of disease. Therefore, we generated transgenic animals expressing the reciprocal fusion RARα-PML cDNA under the control of regulatory sequences derived from the human cathepsin G (hCG) gene, which is preferentially expressed in early myeloid cells.
Here, we report that animals expressing a breakpoint cluster region (bcr)-3-derived isoform of RARα-PML have no alterations in myeloid development, nor do they develop APML. However, when both the PML-RARα and RARα-PML cDNAs are expressed in the early myeloid cells of transgenic mice, the mice display an increased likelihood of developing APML (i.e., increased penetrance of disease), but no significant shortening of the time to disease (latency), suggesting that additional genetic lesions are still required to cause transformation. The phenotype of the APML cells arising in the doubly transgenic animals (designated PR/RP in this study) is similar to that defined previously for hCG-PML-RARα (PR) animals, but the leukemias appear to be less differentiated and perhaps more biologically aggressive. We therefore suggest that RARα-PML potentiates APML development; the 15;17 translocation creates two proteins that contribute to the development of APML, not one.
Materials and Methods
Construction of the Transgene.
We previously have described the generation of the hCG-PML-RARα transgene and PR mice (3). An alternatively spliced bcr-3 isoform of an RARα-PML cDNA was utilized for this study (11). This 1.14-kb cDNA (containing RARα exon 2 fused in frame to PML exons 4–8) was isolated by restriction enzyme digestion with NotI and ligated into the synthetic hCG polylinker at the NotI restriction site. The 7.1-kb hCG-RARα-PML transgene was released from pUC9 sequences (using SalI/BamHI restriction sites) and used for generation of transgenic mice as described previously (12).
Detection of Transgene Expression in Bone Marrow Cells.
RNA was prepared from total bone marrow cells derived from the tibias and femurs of mice. A cDNA of each sample was generated from 1 μg of total cellular RNA by using a reverse transcriptase (RT) step, followed by a PCR, as described previously (3). Briefly, cDNAs were generated by using an RT-PCR kit (Perkin–Elmer). To detect transgene expression, oligonucleotides spanning the junctions of hCG exons 1–2 and 3–2 were used as primers in the PCR. A random primer-labeled hCG exon 2 probe was used to detect the amplified product after transfer to nylon membranes (Hybond, Amersham Pharmacia). cDNAs also were evaluated with murine β-actin primers, as described previously. One-fifth of the actin PCR products were resolved on a 2% agarose gel, transferred to nitrocellulose membrane, and detected with a 32P end-labeled actin-specific oligonucleotide. The same hCG exon 2 probe described above also was used for detecting the PR and RP transgenes in genomic tail DNA digested with EcoRI. To control for DNA content, quality, and loading on the gels, a random primer-labeled granzyme A probe also was hybridized to the nylon membranes.
Generation of PR, RP, and PR/RP Mice.
One PR founder line (135) was bred to two independently derived RP founder mice (2544 and 2683) to generate two intercrossed family cohorts for study. Animals were bred until each cohort contained at least 25 animals with each genotype (i.e., 25 wild-type, RP, PR, and PR/RP animals). Founder lines were maintained by crossing F1 animals with C3H×BL/6 F1 animals, and intercross cohorts were generated by breeding F1 and F2 transgenic animals.
Analysis of Transgenic Animals.
The PR×RP intercrosses were used to generate age-matched family cohorts. Peripheral blood was obtained for complete blood counts and blood differentials. Total spleen weights were documented, and spleen and liver samples were preserved in 10% buffered formalin for histopathologic analysis. Single-cell suspensions of spleen and bone marrow cells were used for flow cytometric and morphologic analyses. Leukemic PR and PR/RP animals were compared by using the same assays.
Cryopreservation and Cell Culture.
Leukemic spleen cells were stored in supplemented DMEM (sDMEM) containing 1% WEHI-3 conditioned medium (WCM) immediately before and after storage; sDMEM medium consists of DMEM, 10% FCS, 10% NCTC-109 (GIBCO/BRL), 0.2 unit/ml insulin (Sigma), 100 units/ml penicillin, 100 mg/ml streptomycin, 25 mM Hepes, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM glutamine, 1 mM oxalacetic acid (Sigma), and 10−5 M 2-mercaptoethanol (Sigma). Fresh APML spleen cells were adjusted to 4 × 107 cells/ml in sDMEM/1% WCM and mixed 1:1 with a 2× freezing medium [sDMEM containing 10% WCM and 20% DMSO (Sigma)]. Multiple samples were stored at 2 × 107 cells/ml in Nunc cryovials and stored at −80°C overnight. Frozen vials were transferred to −198°C for long-term storage. Previously frozen cells were washed and resuspended in PBS before transfer into sex-matched recipient animals (below) or to culture in vitro. Thawed cells were cultured in RPMI containing 20% FCS, 100 units/ml penicillin, 100 μg/ml streptomycin, 5 mM Hepes, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM glutamine, and 3 × 10−6 M 2-mercaptoethanol at 2–3 × 105 cells/ml for 1–3 days (37°C with 5% CO2).
Flow Cytometry.
Single-cell suspensions of spleen, blood, or bone marrow cells were stained with anti-Gr-1 (myeloid differentiation antigen) and either anti-MHC H-2 (Kk or Kb) or anti-murine CD34. Cells were analyzed with single-color staining by using phycoerythrin (PE)- or FITC-conjugated isotype control antibodies to determine specific staining. All antibodies used in this study were purchased from PharMingen and used according to the manufacturer's instructions. For cell sorting experiments, gates were set to include the Gr-1+/CD34− cells or the Gr-1+/CD34+ cells, taking care to exclude cells at the shared boundaries. A minimum of 1 × 105 Gr-1+/CD34+ APML cells were purified by using a 488-nm argon laser on a Beckman Coulter Elite ESP sorting cytometer. Sorted populations and total cells were washed in PBS and used for cytospin and Wright–Giemsa staining.
Transfer of APML Disease to Syngeneic Recipients.
Sex-matched C3H×BL/6 F1 animals were injected i.p. with 105–107 APML spleen cells in 1 ml of PBS. To compare PR and PR/RP APML tumors, C3H×BL/6 F1 recipients were injected with fresh APML spleen cells on the day that cells were harvested. Recipient C3H×BL/6 animals that developed APML became moribund 45–100 days after injection. Recipient animals were scored for APML development on the day of euthanasia or at autopsy by using previously described criteria (3).
Additionally, some APML cells were cryopreserved by using a DMSO-based freezing medium. Cells were brought to 37°C and transferred to 25 ml of sDMEM/1% WCM medium. Cells were washed thoroughly in PBS and injected promptly into C3H×BL/6 F1 recipient mice in 1 ml of PBS. To compare fresh APML spleen cells with cryopreserved cells, spleen cells from four leukemic PR/RP animals were divided into two portions. One was washed in PBS and injected into recipient animals, and the other was cryopreserved in sDMEM medium containing DMSO (see above). Cryopreserved cells were thawed, washed in PBS, and injected into recipient C3H×BL/6 F1 animals after 2 days to 6 months of storage.
Treatment of APML Cells in Vitro.
Previously frozen PR and PR/RP APML spleen cells were cultured for 1–3 days in RPMI/20% FCS in the presence of either 10−6 M AtRA (Sigma) or 0.1% EtOH (AtRA vehicle control). Approximately 104 cells were harvested daily for Wright–Giemsa (Sigma) staining. A minimum of 200 cells were evaluated by two independent observers in a double-blinded fashion. Cultures were analyzed for the percentage of early myeloid cells (blasts, promyelocytes, and myelocytes) vs. late myeloid cells (metamyelocytes, bands, and neutrophils) within each population.
Results
Generation of hCG-RARα-PML (RP) and Doubly Transgenic hCG PML-RARα X hCG-RARα-PML (PR/RP) Transgenic Mice.
We have reported previously the phenotype of transgenic animals expressing the hCG-PML-RARα bcr-1 cDNA (3). To define a biological role for the reciprocal translocation fusion cDNA RARα-PML, we inserted the RARα-PML cDNA into the same hCG regulatory sequences (Fig. 1A). The bcr-3 isoform used in this study contains a fusion of RARα exon 2 in-frame with PML exons 4–8 (11). When this transgene construct was made, the RARα-PML fusion cDNA that complements the bcr-1 PML-RARα cDNA was not available.
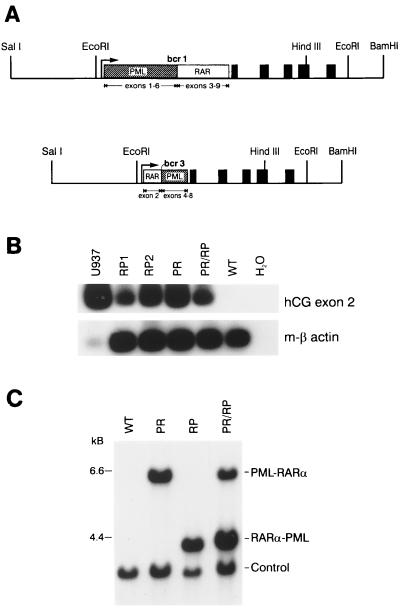
Figure 1.
Construction, expression, and detection of hCG-PML-RARα and hCG RARα-PML transgenes. (A) Diagram of the transgenes. PR and RP cDNAs were ligated into the hCG gene at a synthetic polylinker near the hCG promoter as described previously (2). The 2.5-kb SalI-EcoRI fragment of the transgene contains the 5′ flanking region of the hCG gene. The PML-RARα and RARα-PML cDNAs are shown as boxes and are inserted into the 5′ untranslated region of the hCG gene. The solid boxes represent the five coding exons of hCG. The transgenes also contain the native 3′ flanking sequence of the gene to the BamHI site. (B) RT-PCR analysis of bone marrow RNA derived from PR and RP founder lines and from U937 cells [a human promonocytic cell line that expresses hCG, as a positive control (2)]. Primers spanning hCG exons 1–2 and 3–2 junctions were used to amplify hCG mRNA, as described previously (2). Primers specific for mouse β-actin were used to control for cDNA quality in each sample. The low signal generated in the U937 β-actin lane reflects the specificity of these primers for mouse β-actin. (C) Southern blot detection of transgenes. Tail DNAs were digested with EcoRI and analyzed by Southern blotting. Transgenes were identified with a radiolabeled probe derived from exon 2 of hCG. Doubly transgenic animals contained the expected 6- and 4-kb fragments corresponding to the PR and RP transgenes, respectively. The “control” band is generated by the hybridization of a probe specific for the murine granzyme A gene and serves as a control for DNA loading, transfer, and hybridization.
We identified 10 transgene-positive hCG-RARα-PML animals of 66 potential founders; 5 of these founders were bred, and 4 transmitted the transgene to the germ line. Using RT-PCR, we screened F1 animals from each line for expression of the hCG cassette and selected the two highest-expressing founder lines [2544 (RP1) and 2683 (RP2)] for expansion (Fig. 1B). RP founder lines were bred with one previously characterized, high-expressing PR transgenic line (3) to generate cohorts of animals containing either, both, or neither transgene. EcoRI digestion of genomic tail DNA was used to generate two distinctly sized hCG fragments that we identified with the hCG exon 2 probe, allowing us to screen animals for the presence of either or both transgenes (Fig. 1C).
Expression of RARα-PML Does Not Alter Myeloid Development or Cause APML.
Age-matched RP mice have normal peripheral blood counts and normal percentages of myeloid cells in bone marrow and, therefore, are similar to their wild-type (WT), PR, and PR/RP littermates in this regard (Fig. 2 A and B). Histopathology of RP spleens and livers were analyzed and revealed no evidence of myeloid expansion or granulocytic leukemia (n = 25). Indeed, >40 RP animals have been aged >2 years and developed no clinical or histopathologic evidence of APML. Thus, expression of the reciprocal RARα-PML bcr-3 under hCG regulatory sequences is not sufficient to cause APML in transgenic mice.
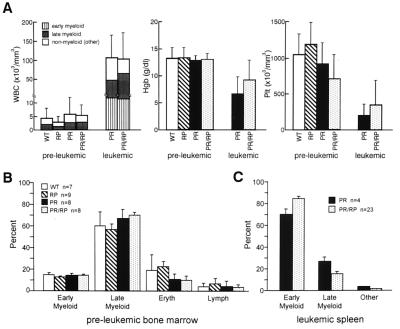
Figure 2.
Hematologic analysis of young transgenic animals. (A) Comparison of peripheral blood counts. Blood was obtained from six cohorts of healthy age-matched, genotyped littermates at 2–9 months of age. Values represent the mean ± SD of total WBC (expressed as cells/mm3), hemoglobin (Hgb, expressed as gm/dl), and platelets (Plt, expressed as Plt × 103/mm3). PR and PR/RP leukemic animals (n = 4 for each) were also analyzed. (B) Comparison of bone marrow differentials from healthy young animals. Differential counts of at least 150 cells were performed by two independent observers blinded to genotypes. Values reflect the mean ± SD, indicated by error bars. Early myeloid cells include blasts, promyelocytes, and myelocytes, and late myeloid cells include metamyelocytes, band, and neutrophils. “Eryth” includes all erythroid precursors. (C) Comparison of differential counts from the leukemic spleens of PR vs. PR/RP transgenic mice. More than 95% of the cells in all spleens were myeloid. Early myeloid cells and late myeloid cells were defined as described above. The percentage of early myeloid cells is significantly higher (P < 0.05) in the PR/RP-derived spleens.
Expression of RARα-PML Increases the Penetrance of APML in PML-RARα Transgenic Mice but Does Not Alter Latency.
Two large, transgenic families were generated by breeding one PR founder with two independently derived RP founders until >25 animals from each genotype (RP, PR, PR/RP, and WT) were obtained; these animals were monitored for the development of APML. Littermates of each genotype were analyzed at several time points in the study. Histologic analysis of young, preleukemic PR and PR/RP mice revealed increased numbers of early myeloid cells in the splenic red pulp in virtually every animal, as described previously (3). In addition, PR and PR/RP spleens were enlarged significantly (P < 0.0001) in virtually all preleukemic animals less than 8 months of age [PR = 0.17 g ± 0.03 (n = 9) and PR/RP = 0.18 g ± 0.05 (n = 9)] compared with RP and WT mice [RP = 0.10 g ± 0.02 (n = 9) and WT = 0.11 g ± 0.02 (n = 10)].
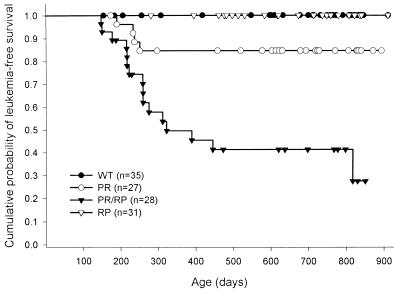
In the first completed intercross, APML developed in 0 of 35 WT animals, 0 of 31 RP animals, 4 of 27 (15%) PR animals, and 16 of 28 (57%) PR/RP animals after 2 years of observation (P = 0.0005, Fig. 3). The average time to development of disease was 7.5 ± 0.8 months for the PR mice vs. 9.7 ± 5.2 months for the PR/RP mice. The second intercross was less mature than the first, but the results were similar; APML developed in 0 of 37 RP animals, 1 of 31 PR animals, 0 of 30 RP animals, and 7 of 36 PR/RP animals after 12 months of observation, on average. The time to APML development in the second-intercross PR/RP animals was 12.7 ± 3.3 months, which is not statistically different than the time to APML development in the first intercross (P > 0.1). Because both intercrosses demonstrate increased APML penetrance in the doubly transgenic animals, it is unlikely that the action of RARα-PML is caused by an integration site-specific effect of the RP transgene.
Figure 3.
Kaplan–Meier analysis of APML disease in transgenic animals. One PR founder (135) was bred with two independently derived RP founders (2544 and 2683) to establish intercross cohorts. Animals were scored for development of APML (using criteria described previously; see ref. 3) when sacrificed or autopsied. Data represent the complete study of the first intercross (PR 135 × RP1 2544). The cumulative probability of death resulting from APML at 24 months is significantly higher in the PR/RP animals compared with the PR animals (P = 0.0005).
The Phenotypes of Leukemic Cells Derived from PR/RP vs. PR Animals.
Peripheral blood from 11 PR (some from the first intercross and some from simultaneous breedings) and 16 PR/RP leukemic animals was analyzed. The mean total white blood cell counts were elevated similarly, and the hemoglobin concentrations and platelet counts were depressed similarly (Fig. 2A). The percentage of early myeloid cells in the peripheral blood (Fig. 2A) was similar. The spleens of leukemic animals contained >95% myeloid cells in both PR- and PR/RP-derived tumors. Differentials of leukemic spleen preparations from PR animals revealed that 70 ± 5% of the total cells were promyelocytes and myelocytes (n = 4) (Fig. 2C), which is comparable to bone marrow differentials obtained from these animals in this study (68 ± 7% promyelocytes and myelocytes) and in a previous one (see ref. 3). Leukemic spleen cells from PR/RP animals contained 84 ± 2% promyelocytes and myelocytes (n = 23, P < 0.05) (Fig. 2C), which demonstrates that the PR/RP-derived tumors contained a higher percentage of immature myeloid cells than the PR-derived tumors.
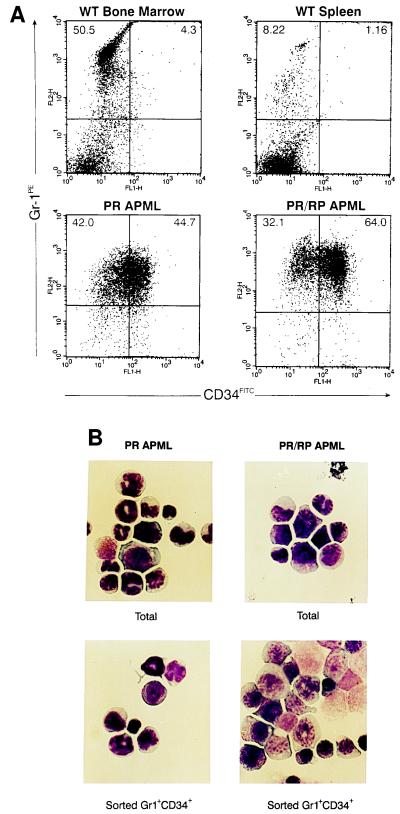
We next analyzed several hematopoietic surface antigens present on the spleen, bone marrow, and blood cells derived from leukemic animals. Leukemic spleen cells from PR/RP and PR mice were uniformly positive for the myeloid differentiation marker Gr-1, as noted previously (4). In addition, 2 of 2 PR APML and 16 of 16 PR/RP leukemic spleens contained an abnormal population of Gr-1+ cells that was also CD34+ (Fig. 4A); these Gr-1+/CD34+ cells were also found in the bone marrow and blood of all leukemic animals tested, but were not detected in several wild-type bone marrow and spleen preparations (Fig. 4 and data not shown). Two PR-derived leukemic spleens were analyzed and contained 34% and 45% Gr-1+/CD34+ cells; 15 PR/RP leukemic spleens were analyzed, and these contained 55 ± 13% Gr-1+/CD34+ cells (with a range of 28–67%). However, neither PR nor PR/RP leukemic spleen cells demonstrated expression of another early hematopoietic marker, Sca-1 (zero of nine tested, data not shown). Similarly, none of the leukemic cells stained positive for FITC-conjugated, isotype-matched antibodies against the B/natural killer cell marker B220 or the control antigen DNP. We used flow cytometry to isolate Gr-1+/CD34− vs. Gr-1+/CD34+ cells for morphologic evaluation (Fig. 4B) and found that the Gr-1+/CD34+ cells were enriched for immature myeloid cells.
Figure 4.
Flow cytometric analysis of APML cells. (A) Flow cytometric plots of WT bone marrow and spleen cells compared with PR and PR/RP APML spleen cells. All cells were stained for myeloid surface antigen Gr-1 (Gr-1PE) and the early hematopoietic progenitor marker CD34 (CD34FITC). The indicated gates were used to determine the percentage of cells positive for Gr-1 vs. Gr-1+/CD34+. (B) APML spleen cells were analyzed by Wright–Geimsa staining before and after flow cytometric sorting. Note that Gr-1+/CD34+ populations are enriched for early myeloid cells.
Because a recent report suggested that human PML-RARα may down-regulate MHC class I expression (13), we tested 18 leukemic spleen samples [derived from both PR (n = 2) and PR/RP mice (n = 16)] for surface expression of MHC class I molecules. Because our intercrossed animals were generated on a C3H×C57BL/6 background, APML cells should display both H-2k (from C3H) and H-2b (from BL/6) class I MHC markers. Most Gr-1+ PR/RP and PR leukemic spleen cells (16 of 18 tumors tested) were clearly positive for the surface expression of class I by using these antibodies. The level of class I expression on APML cells essentially was equivalent to the levels detected on Gr-1+ cells derived from normal bone marrow or spleen (data not shown).
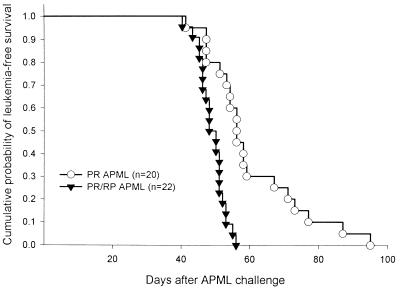
We and others have shown previously that transfer of APML cells into severe combined immunodeficient and syngeneic recipients uniformly causes fatal APML (3, 4). We wished to determine whether PR vs. PR/RP APML spleen cells differed in their ability to initiate leukemia in syngeneic C3H×BL/6 F1 recipients. We therefore harvested leukemic spleen cells from 3 leukemic PR mice and 3 leukemic PR/RP mice and injected i.p. 1–3 × 106 tumor cells (in 1 ml of PBS) into 6–10 recipient animals for each tumor. Both PR and PR/RP APML spleen cells were able to cause fatal APML in 100% of naïve, nonirradiated C3H×BL/6 F1 animals; however, PR/RP APML cells caused death more quickly than identical doses of PR-derived APML cells (Fig. 5, P < .001).
Figure 5.
PR/RP leukemic spleen cells cause death in syngeneic recipients more quickly than PR cells. Each of three independent PR and PR/RP APML tumors were transferred into 20–22 C3H×BL/6 F1 recipients at 1–3 × 106 cells per mouse. Animals were scored for APML on the day of sacrifice or at autopsy, and outcomes were plotted by using a Kaplan–Meier analysis. The difference between the time of death caused by PR vs. PR/RP leukemic spleen cells was highly significant (P < 0.001).
PR- and PR/RP-Derived Leukemic Cells Both Respond to AtRA.
To study APML spleen cells in therapy protocols, we optimized a standard method for cryopreservation in a DMSO-based medium. Previously frozen PR and PR/RP APML cells retain the same patterns and levels of cell surface antigens Gr-1, MHC class I, and CD34 as freshly harvested cells (five of five tumors tested; data not shown). Comparison of fresh vs. cryopreserved cells also has shown no difference in the morphology of leukemic spleens (not shown) or the ability to transfer APML to C3H×BL/6 F1 recipients at several defined doses of spleen cells (data not shown).
Two cryopreserved PR leukemic spleen samples and three PR/RP samples were incubated for 3 days in the presence of 10−6 M AtRA or its vehicle and then assessed morphologically for differentiation (Table 1). All samples contained significantly higher percentages of terminally differentiated cells when cultured in the presence of 10−6 M AtRA, although the absolute magnitude of the AtRA effect varied from tumor to tumor. Of note, all of the leukemias demonstrated spontaneous differentiation in our culture conditions (RPMI with 20% FCS), with or without the AtRA vehicle.
Table 1.
Leukemic spleen cells from PR and PR/RP animals respond to AtRA in vitro
| Day 0
|
Day 3
|
||
|---|---|---|---|
| No treatment | Control/vehicle | AtRA | |
| PRAPML 1 | 45 ± 3 | 61 ± 4 | 86 ± 1* |
| PRAPML 2 | 36 ± 2 | 65 ± 7 | 85 ± 3* |
| PR/RPAPML 1 | 4 ± 1 | 45 ± 3 | 72 ± 1* |
| PR/RPAPML 2 | 15 ± 2 | 45 ± 3 | 69 ± 5* |
| PR/RPAPML 3 | 12 ± 1 | 40 ± 3 | 83 ± 1* |
Cryopreserved APML spleen cells were cultured for 3 days in the presence of 10−6 M AtRA and compared with AtRA vehicle and day 0 control cultures. Cells were analyzed for morphologic differentiation, and the percentage of late myeloid forms (metamyelocytes, band forms, and mature neutrophils) were scored by two independent observers blinded to the treatment arm. Each sample was treated in three separate experiments, and the results are shown as the average percentage of late myeloid cells within the total population ± SEM. Significant differences in the percentages of mature cells, compared with day 3 vehicle, are designated with an asterisk.
Discussion
In this report, we generated and compared transgenic mice expressing a PML-RARα fusion cDNA, an RARα-PML fusion cDNA, or both cDNAs under the control of hCG regulatory sequences. RP transgenic animals have normal myeloid development and do not develop myeloid leukemia, even after prolonged periods of observation. However, coexpression of RARα-PML with PML-RARα significantly increased the penetrance of APML development in PR/RP animals, but it did not reduce latency. The leukemic spleen cells derived from PR/RP animals appeared to contain a higher percentage of immature cells and caused death in secondary recipients more quickly than leukemic cells derived from PR animals.
Previous studies have reported that PML-RARα is expressed in 100% of APML patients bearing the (15;17) (q22; q11.2) translocation, but the reciprocal fusion cDNA is detected in only 70–80% of patients (7–10). Because the RARα promoter and transcription/translation start sites are the same for all of the reciprocal fusion genes (see below), there is no clear-cut explanation for this finding. Because RARα is expressed at extremely low levels in myeloid cells, it is difficult to rule out the possibility that the leukemic cells bearing RP transcripts were present, but not adequately enriched for detection in some patients. Of note, all cases of variant APML [t(11;17) PLZF-RARα and the case report of t(5;17) NPM-RARα] demonstrate expression of their reciprocal RARα fusion cDNAs (14–17). Despite the expression of the reciprocal fusion cDNAs in most (if not all) of these patients, there have been no systematic studies of the contribution of RARα-PML to the APML phenotype, perhaps because the reciprocal fusion has not been thought to contain any of the critical functional domains of either protein.
Translocations involving PML and RARα nearly always occur between intron 2 of RARα and one of three locations within the PML gene (reviewed in ref. 2). The most common PML/RARα translocation (bcr-1) results in the L form, where the PML breakpoint occurs in intron 6. The bcr-1 translocation fuses exons 1–6 of PML in-frame with exon 3 of RARα, yielding a fusion cDNA that contains nearly all of the known functional domains of both PML and RARα. Translocations occurring in PML intron 3 (S form or bcr-3) result in a longer reciprocal fusion protein that contains one of the PML zinc finger-like domains (8). In one study, patients bearing the bcr-3 form of the translocation were found to have higher total WBC counts and more immature cells at presentation, but similar rates of complete remission and overall survival (18). A second study of patients with bcr-1 vs. bcr-3 did not detect any differences in the APML phenotype, in the expression of the reciprocal cDNAs, or in responses to AtRA or chemotherapy (8). However, a role for the RARα-PML fusion protein in APML development was suggested strongly by the identification of an APML patient whose leukemic cells carried a microinsertion of PML genetic material into RARα, yielding the chimeric fusion protein RARα-PML bcr-3, but no detectable PML-RARα fusion (19). In this study, we chose to express an isoform of the bcr-3 reciprocal cDNA because it was available, because it contained the greatest amount of PML sequence, and because it had been associated independently with APML development in the single case of the microinsertion. The bcr-3 RARα-PML cDNA is not the true reciprocal of the bcr-1-derived PML-RARα cDNA used in all of the transgenic studies described to date (3–5), but it still complements its function in this model system, as described below. Another experiment with the bcr-1-derived reciprocal cDNA is in progress, but it will require several more years to complete.
RARα-PML appears to potentiate the leukemogenicity of PML-RARα. One potential explanation for this observation is that RARα-PML may behave as a dominant negative inhibitor of PML. PML may affect cell proliferation, differentiation, and tumor suppression (20, 21), which suggests that PML loss of function could increase the penetrance of APML. Alternatively, RARα-PML potentially could act to sequester and inhibit the retinoblastoma protein (Rb). PML colocalizes with the nonphosphorylated fraction of Rb in the nuclear bodies; PML binds Rb via its B boxes and its C-terminal domain (22), which is retained in the PML portion of bcr-3 fusion used in this study. It therefore is possible that the PML portion of RARα-PML may bind to and inactivate Rb, but we do not yet know how this might contribute to leukemogenesis. Rb controls cell proliferation by regulating the E2F transcription factors (which activate genes at the G1/S transition) (23). The sequestration of Rb therefore could release E2F family members from inhibition, which would tend to drive cells into S phase. The sequestration of Rb by RARα-PML also potentially could affect myeloid differentiation. Although Rb is not known currently to play a role in myeloid development, it does have effects on muscle cell differentiation (24) and it plays a role in chicken erythroid development by regulating the glucocorticoid receptor in erythroid progenitors (25). Clearly, further work will be required to establish the mechanism by which RARα-PML potentiates APML development and to determine whether it synergizes only with PML-RARα.
Using a variety of assays, we compared the phenotypes of preleukemic and leukemic animals expressing PML-RARα alone vs. animals expressing both transgenes. Preleukemic animals are indistinguishable, but leukemias from the doubly transgenic animals appear to contain a larger percentage of immature myeloid cells. In addition, when equal numbers of leukemic spleen cells are transferred to syngeneic recipients, the doubly transgenic cells cause APML more quickly. These results suggest that the leukemias arising in the doubly transgenic animals may be more biologically aggressive and/or they may contain a higher fraction of leukemia-initiating cells.
We examined PR- and PR/RP APML-derived spleen cells to determine whether AtRA-induced myeloid differentiation was altered by the expression of RARα-PML. Myeloid differentiation was induced by AtRA in both the PR- and PR/RP-derived leukemias, suggesting that PML-RARα itself is the major target of AtRA-induced differentiation. Therefore, RARα-PML bcr-3 does not seem to exert a direct effect on retinoid signaling via the PML-RARα fusion protein in vitro.
Using flow cytometry, we found that the leukemic spleen cells from both PR and PR/RP mice contain a large population of abnormal Gr-1+/CD34+ cells. This finding was unexpected, because CD34 expression frequently is seen in human cases of AML M1, M2 t(8;21), and M4 (inversion 16), but not in classical APML (M3) (26) and because CD34+ cells derived from the bone marrow of APML patients appear to be negative for PML-RARα transcripts (27). However, CD34 expression recently was detected on the leukemic cells of 71% of “hypogranular” M3 and M3v patients carrying t(15;17) (28). The leukemic spleen cells from our mice were uniformly positive for the myeloid differentiation antigen Gr-1 and for CD34, but they were uniformly negative for expression of Sca-1, another early hematopoietic antigen. At this time, we do not know whether CD34 expression on these leukemic cells is due to direct activation of the CD34 gene by a transcriptional effect of PML-RARα or whether it represents an alteration in myeloid differentiation that is caused by PML-RARα expression in early myeloid cells. Additional studies will be required to resolve this issue.
In summary, we have determined that one isoform of a reciprocal fusion partner of the PML-RARα oncogene can potentiate the development of APML. Our results do not define the mechanism by which RARα-PML causes this effect, but they do suggest that RARα-PML acts in conjunction with PML-RARα. These data demonstrate a biological function for RARα-PML and they create a strong rationale for defining the mechanism that causes it to contribute to the development of this disease.
Acknowledgments
We thank Pam Goda and Kelly Schrimpf for excellent care of these animals and Nancy Reidelberger for expert preparation of the manuscript. We acknowledge Jeffrey Haug for his time and expertise in the sorting experiments and Andrew Lane for his contributions to this manuscript. This work was supported by National Institutes of Health Grant CA49712 (T.J.L.).
Abbreviations
- APML
acute promyelocytic leukemia
- RAR
retinoic acid receptor
- hCG
human cathepsin G
- bcr
breakpoint cluster region
- RT-PCR
reverse transcriptase–PCR
- sDMEM
supplemented DMEM
- WT
wild type
- PR
hCG-PML-RARα
- RP
RARα-PML
- Rb
retinoblastoma protein
References
- 1.Lo Coco F, Diverio D, Falini B, Biondi A, Nervi C, Pelicci P G. Blood. 1999;94:12–22. [PubMed] [Google Scholar]
- 2.Melnick A, Licht J D. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 3.Grisolano J L, Wesselschmidt R L, Pelicci P G, Ley T J. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- 4.Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci P G, Atwater S, Bishop J M. Proc Natl Acad Sci USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L-Z, Tribioli C, Rivi R, Peruzzi D, Pelicci P G, Soares V, Cattoretti G, Pandolfi P P. Proc Natl Acad Sci USA. 1997;94:5302–5307. doi: 10.1073/pnas.94.10.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trecca D, Longo L, Biondi A, Cro L, Calori R, Grignani F, Maiolo A T, Pelicci P G, Neri A. Am J Hematol. 1994;46:304–309. doi: 10.1002/ajh.2830460409. [DOI] [PubMed] [Google Scholar]
- 7.Alcalay M, Zangrilli D, Fagioli M, Pandolfi P P, Mencarelli A, Lo Coco F, Biondi A, Grignani F, Pelicci P G. Proc Natl Acad Sci USA. 1992;89:4840–4844. doi: 10.1073/pnas.89.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y-P, Andersen J, Zelent A, Rao S, Paietta E, Tallman M S, Wiernik P H, Gallagher R E. Blood. 1997;90:306–312. [PubMed] [Google Scholar]
- 9.Grimwade D, Howe K, Langabeer S, Davies L, Oliver F, Walker H, Swirsky D, Wheatley K, Goldstone A, Burnett A, et al. Br J Haematol. 1996;94:557–573. [PubMed] [Google Scholar]
- 10.Borrow J, Goddard A D, Gibbons B, Katz F, Swirsky D, Fioretos T, Dube I, Winfield D A, Kingston J, Hagemeijer A, et al. Br J Haematol. 1992;82:529–540. doi: 10.1111/j.1365-2141.1992.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 11.Fagioli M, Alcalay M, Pandolfi P P, Venturini L, Mencarelli A, Simeone A, Acampora D, Grignani F, Pelicci P G. Oncogene. 1992;7:1083–1091. [PubMed] [Google Scholar]
- 12.Grisolano J L, Sclar G M, Ley T J. Proc Natl Acad Sci USA. 1994;91:8989–8993. doi: 10.1073/pnas.91.19.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng P, Guo Y, Niu Q, Levy D E, Dyck J A, Lu S, Sheiman L A, Liu Y. Nature (London) 1998;396:373–376. doi: 10.1038/24628. [DOI] [PubMed] [Google Scholar]
- 14.Li J-Y, English M A, Ball H J, Yeyati P L, Waxman S, Licht J D. J Biol Chem. 1997;272:22447–22455. doi: 10.1074/jbc.272.36.22447. [DOI] [PubMed] [Google Scholar]
- 15.Licht J D, Shaknovich R, English M A, Melnick A, Li J-Y, Reddy J C, Dong S, Chen S-J, Zelent A, Waxman S. Oncogene. 1996;12:323–336. [PubMed] [Google Scholar]
- 16.Grimwade D, Gorman P, Duprez E, Howe K, Langabeer S, Oliver F, Walker H, Culligan D, Waters J, Pomfret M, et al. Blood. 1997;90:4976–4885. [PubMed] [Google Scholar]
- 17.Redner R L, Rush E A, Faas S, Rudert W A, Corey S J. Blood. 1996;87:882–886. [PubMed] [Google Scholar]
- 18.Gallagher R E, Willman C L, Slack J L, Andersen J W, Li Y-P, Viswanatha D, Bloomfield C D, Appelbaum F R, Schiffer C A, Tallman M S, et al. Blood. 1997;90:1656–1663. [PubMed] [Google Scholar]
- 19.Lafage-Pochitaloff M, Alcalay M, Brunel V, Longo L, Sainty D, Simonetti J, Birg F, Pelicci P G. Blood. 1995;85:1169–1174. [PubMed] [Google Scholar]
- 20.Wang Z G, Delva L, Gaboli M, Rivi R, Giorgio M, Cordon-Cardo C, Grosveld F, Pandolfi P P. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 21.Liu J H, Mu Z M, Chang K S. J Exp Med. 1995;181:1965–1973. doi: 10.1084/jem.181.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcalay M, Tomassoni L, Colombo E, Stoldt S, Grignani F, Fagioli M, Szekely L, Helin K, Pelicci P G. Mol Cell Biol. 1998;18:1084–1093. doi: 10.1128/mcb.18.2.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 24.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 25.Wessely O, Deiner E M, Beug H, von Lindern M. EMBO J. 1997;16:267–280. doi: 10.1093/emboj/16.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause D S, Fackler M J, Civin C I, May W S. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 27.Turhan A G, Lemoine F M, Debert C, Bonnet M L, Baillou C, Picard F, Macintyre E A, Varet B. Blood. 1995;85:2154–2161. [PubMed] [Google Scholar]
- 28.Piedras J, Lopez-Karpovitch X, Cardenas R. Cytometry. 1998;32:286–290. [PubMed] [Google Scholar]